Role of Alternative Elicitor Transporters in the Onset of Plant Host Colonization by Streptomyces scabiei 87-22
- PMID: 36829511
- PMCID: PMC9953190
- DOI: 10.3390/biology12020234
Role of Alternative Elicitor Transporters in the Onset of Plant Host Colonization by Streptomyces scabiei 87-22
Abstract
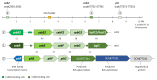
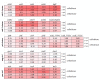
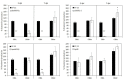
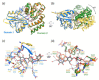
Plant colonization by Streptomyces scabiei, the main cause of common scab disease on root and tuber crops, is triggered by cello-oligosaccharides, cellotriose being the most efficient elicitor. The import of cello-oligosaccharides via the ATP-binding cassette (ABC) transporter CebEFG-MsiK induces the production of thaxtomin phytotoxins, the central virulence determinants of this species, as well as many other metabolites that compose the 'virulome' of S. scabiei. Homology searches revealed paralogues of the CebEFG proteins, encoded by the cebEFG2 cluster, while another ABC-type transporter, PitEFG, is encoded on the pathogenicity island (PAI). We investigated the gene expression of these candidate alternative elicitor importers in S. scabiei 87-22 upon cello-oligosaccharide supply by transcriptomic analysis, which revealed that cebEFG2 expression is highly activated by both cellobiose and cellotriose, while pitEFG expression was barely induced. Accordingly, deletion of pitE had no impact on virulence and thaxtomin production under the conditions tested, while the deletion of cebEFG2 reduced virulence and thaxtomin production, though not as strong as the mutants of the main cello-oligosaccharide transporter cebEFG1. Our results thus suggest that both ceb clusters participate, at different levels, in importing the virulence elicitors, while PitEFG plays no role in this process under the conditions tested. Interestingly, under more complex culture conditions, the addition of cellobiose restored thaxtomin production when both ceb clusters were disabled, suggesting the existence of an additional mechanism that is involved in sensing or importing the elicitor of the onset of the pathogenic lifestyle of S. scabiei.
Keywords: Streptomyces scabiei; cello-oligosaccharide; cellulose utilization; elicitor sensing; phytotoxin; plant pathogen; secondary metabolism; sugar transport; virulence; virulome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The virulome of Streptomyces scabiei in response to cello-oligosaccharide elicitors.Microb Genom. 2022 Jan;8(1):000760. doi: 10.1099/mgen.0.000760. Microb Genom. 2022. PMID: 35040428 Free PMC article.
-
The CebE/MsiK Transporter is a Doorway to the Cello-oligosaccharide-mediated Induction of Streptomyces scabies Pathogenicity.Sci Rep. 2016 Jun 2;6:27144. doi: 10.1038/srep27144. Sci Rep. 2016. PMID: 27250236 Free PMC article.
-
Proteomic Response to Thaxtomin Phytotoxin Elicitor Cellobiose and to Deletion of Cellulose Utilization Regulator CebR in Streptomyces scabies.J Proteome Res. 2018 Nov 2;17(11):3837-3852. doi: 10.1021/acs.jproteome.8b00528. Epub 2018 Oct 10. J Proteome Res. 2018. PMID: 30229651
-
Genetic and physiological determinants of Streptomyces scabies pathogenicity.Mol Plant Pathol. 2009 Sep;10(5):579-85. doi: 10.1111/j.1364-3703.2009.00561.x. Mol Plant Pathol. 2009. PMID: 19694949 Free PMC article. Review.
-
Regulation of virulence mechanisms in plant-pathogenic Streptomyces.Can J Microbiol. 2024 Jun 1;70(6):199-212. doi: 10.1139/cjm-2023-0171. Epub 2024 Jan 8. Can J Microbiol. 2024. PMID: 38190652 Review.
Cited by
-
Editorial for the Special Issue, 'Secondary Metabolites from Microorganisms or Microorganism-Host Interaction?'.Biology (Basel). 2023 Dec 12;12(12):1515. doi: 10.3390/biology12121515. Biology (Basel). 2023. PMID: 38132341 Free PMC article.
-
Physiological Functions of the Cello-Oligosaccharides Binding CebE in the Pathogenic Streptomyces sp. AMCC400023.Microorganisms. 2024 Feb 29;12(3):499. doi: 10.3390/microorganisms12030499. Microorganisms. 2024. PMID: 38543550 Free PMC article.
-
Tumescenamide C, a cyclic lipodepsipeptide from Streptomyces sp. KUSC_F05, exerts antimicrobial activity against the scab-forming actinomycete Streptomyces scabiei.J Antibiot (Tokyo). 2024 Jun;77(6):353-364. doi: 10.1038/s41429-024-00716-4. Epub 2024 Mar 25. J Antibiot (Tokyo). 2024. PMID: 38523145
References
-
- Johnson E.G., Joshi M.V., Gibson D.M., Loria R. Cello-oligosaccharides released from host plants induce pathogenicity in scab-causing Streptomyces species. Physiol. Mol. Plant Pathol. 2007;71:18–25. doi: 10.1016/j.pmpp.2007.09.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

