Reduced Renal CSE/CBS/H2S Contributes to the Progress of Lupus Nephritis
- PMID: 36829595
- PMCID: PMC9953544
- DOI: 10.3390/biology12020318
Reduced Renal CSE/CBS/H2S Contributes to the Progress of Lupus Nephritis
Abstract
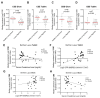
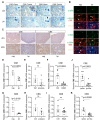
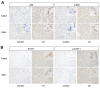
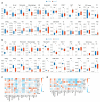
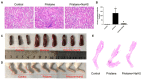
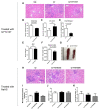
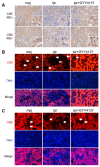
The molecular mechanisms underlying lupus nephritis (LN) pathogenesis are not fully understood. Hydrogen sulfide (H2S) is involved in many pathological and physiological processes. We sought to investigate the roles of H2S in LN pathogenesis. H2S synthase cystathionine-lyase (CSE) and cystathionine-synthetase (CBS) expression was downregulated in renal tissues of patients with LN and their levels were associated with LN's prognosis using the Nephroseq database. Reduced CSE and CBS protein expression in kidney tissues of LN patients and MRL/lpr mice were confirmed by immunohistochemistry. CSE and CBS mRNA levels were reduced in MRL/lpr and pristine- and R848-induced lupus mice. Given that H2S exerts an anti-inflammatory role partly via regulating inflammatory transcription factors (TFs), we analyzed hub TFs by using a bioinformatics approach. It showed that STAT1, RELA, and T-cell-related signaling pathways were enriched in LN. Increased STAT1 and RELA expression were confirmed in renal tissues of LN patients. Treatment of MRL/lpr and pristine mice with H2S donors alleviated systemic lupus erythematosus (SLE) phenotypes and renal injury. H2S donors inhibited RELA level and T-cell infiltration in the kidneys of MRL/lpr and pristine mice. Our data indicated that CSE/CBS/H2S contributes to LN pathogenesis. Supplementation of H2S would be a potential therapeutic strategy for LN.
Keywords: CBS; CSE; H2S; RELA; STAT1; T cells; lupus nephritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Complement factor B inhibitor LNP023 improves lupus nephritis in MRL/lpr mice.Biomed Pharmacother. 2022 Sep;153:113433. doi: 10.1016/j.biopha.2022.113433. Epub 2022 Jul 26. Biomed Pharmacother. 2022. PMID: 36076550
-
Dysregulation of cystathionine γ-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage.Cell Signal. 2013 Nov;25(11):2255-62. doi: 10.1016/j.cellsig.2013.07.010. Epub 2013 Jul 18. Cell Signal. 2013. PMID: 23872072
-
1,25-dihydroxyvitamin D3 ameliorates lupus nephritis through inhibiting the NF-κB and MAPK signalling pathways in MRL/lpr mice.BMC Nephrol. 2022 Jul 8;23(1):243. doi: 10.1186/s12882-022-02870-z. BMC Nephrol. 2022. PMID: 35804318 Free PMC article.
-
The CBS/CSE system: a potential therapeutic target in NAFLD?Can J Physiol Pharmacol. 2015 Jan;93(1):1-11. doi: 10.1139/cjpp-2014-0394. Can J Physiol Pharmacol. 2015. PMID: 25493326 Review.
-
Targeting hydrogen sulfide as a promising therapeutic strategy for atherosclerosis.Int J Cardiol. 2014 Mar 15;172(2):313-7. doi: 10.1016/j.ijcard.2014.01.068. Epub 2014 Jan 24. Int J Cardiol. 2014. PMID: 24491853 Review.
Cited by
-
Complex Pathophysiology of Acute Kidney Injury (AKI) in Aging: Epigenetic Regulation, Matrix Remodeling, and the Healing Effects of H2S.Biomolecules. 2024 Sep 17;14(9):1165. doi: 10.3390/biom14091165. Biomolecules. 2024. PMID: 39334931 Free PMC article. Review.
References
Grants and funding
- No.82200812 and 81974090/National Natural Science Foundation of China
- No.2022JJ30968/Natural Science Foundation of Hunan province
- No.kq2202371/Natural Science Foundation of Changsha city
- No.2022M710171/Post-doctoral program
- No.2022ZZTS0940/Innovation of science and technology of Central South University
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

