Vascularized Tissue Organoids
- PMID: 36829618
- PMCID: PMC9951914
- DOI: 10.3390/bioengineering10020124
Vascularized Tissue Organoids
Abstract
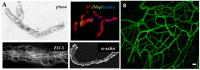
Tissue organoids hold enormous potential as tools for a variety of applications, including disease modeling and drug screening. To effectively mimic the native tissue environment, it is critical to integrate a microvasculature with the parenchyma and stroma. In addition to providing a means to physiologically perfuse the organoids, the microvasculature also contributes to the cellular dynamics of the tissue model via the cells of the perivascular niche, thereby further modulating tissue function. In this review, we discuss current and developing strategies for vascularizing organoids, consider tissue-specific vascularization approaches, discuss the importance of perfusion, and provide perspectives on the state of the field.
Keywords: endothelial; extracellular matrix; microvessel; organoid; perfusion; spheroid; stem cell; vascular network; vascularization; vessel.
Conflict of interest statement
J.B.H. is a partner of Advanced Solutions. H.A.S., S.M.M. and J.B.H. are employees at Advanced Solutions.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources

