An In Vivo Platform for Rebuilding Functional Neocortical Tissue
- PMID: 36829757
- PMCID: PMC9952056
- DOI: 10.3390/bioengineering10020263
An In Vivo Platform for Rebuilding Functional Neocortical Tissue
Abstract
Recent progress in cortical stem cell transplantation has demonstrated its potential to repair the brain. However, current transplant models have yet to demonstrate that the circuitry of transplant-derived neurons can encode useful function to the host. This is likely due to missing cell types within the grafts, abnormal proportions of cell types, abnormal cytoarchitecture, and inefficient vascularization. Here, we devised a transplant platform for testing neocortical tissue prototypes. Dissociated mouse embryonic telencephalic cells in a liquid scaffold were transplanted into aspiration-lesioned adult mouse cortices. The donor neuronal precursors differentiated into upper and deep layer neurons that exhibited synaptic puncta, projected outside of the graft to appropriate brain areas, became electrophysiologically active within one month post-transplant, and responded to visual stimuli. Interneurons and oligodendrocytes were present at normal densities in grafts. Grafts became fully vascularized by one week post-transplant and vessels in grafts were perfused with blood. With this paradigm, we could also organize cells into layers. Overall, we have provided proof of a concept for an in vivo platform that can be used for developing and testing neocortical-like tissue prototypes.
Keywords: layering; neocortex; tissue replacement; transplant; vascularization.
Conflict of interest statement
J.M.H. is Founder of BE Therapeutics Inc., a company aimed at brain tissue repair. There are no other conflicts to report.
Figures
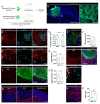

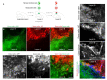



References
-
- Linaro D., Vermaercke B., Iwata R., Ramaswamy A., Libé-Philippot B., Boubakar L., Davis B.A., Wierda K., Davie K., Poovathingal S., et al. Xenotransplanted Human Cortical Neurons Reveal Species-Specific Development and Functional Integration into Mouse Visual Circuits. Neuron. 2019;104:972–986.e6. doi: 10.1016/j.neuron.2019.10.002. - DOI - PMC - PubMed
-
- Espuny-Camacho I., Michelsen K.A., Linaro D., Bilheu A., Acosta-Verdugo S., Herpoel A., Giugliano M., Gaillard A., Vanderhaeghen P. Human Pluripotent Stem-Cell-Derived Cortical Neurons Integrate Functionally into the Lesioned Adult Murine Visual Cortex in an Area-Specific Way. Cell Rep. 2018;23:2732–2743. doi: 10.1016/j.celrep.2018.04.094. - DOI - PMC - PubMed
-
- Michelsen K.A., Acosta-Verdugo S., Benoit-Marand M., Espuny-Camacho I., Gaspard N., Saha B., Gaillard A., Vanderhaeghen P. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron. 2015;85:982–997. doi: 10.1016/j.neuron.2015.02.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

