Aging-Associated Thyroid Dysfunction Contributes to Oxidative Stress and Worsened Functional Outcomes Following Traumatic Brain Injury
- PMID: 36829776
- PMCID: PMC9952686
- DOI: 10.3390/antiox12020217
Aging-Associated Thyroid Dysfunction Contributes to Oxidative Stress and Worsened Functional Outcomes Following Traumatic Brain Injury
Abstract
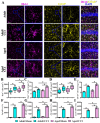
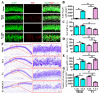
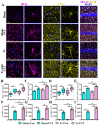
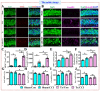
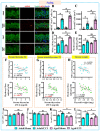
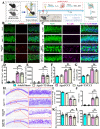
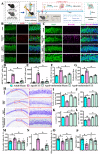
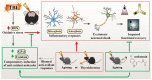
The incidence of traumatic brain injury (TBI) increases dramatically with advanced age and accumulating evidence indicates that age is one of the important predictors of an unfavorable prognosis after brain trauma. Unfortunately, thus far, evidence-based effective therapeutics for geriatric TBI is limited. By using middle-aged animals, we first confirm that there is an age-related change in TBI susceptibility manifested by increased inflammatory events, neuronal death and impaired functional outcomes in motor and cognitive behaviors. Since thyroid hormones function as endogenous regulators of oxidative stress, we postulate that age-related thyroid dysfunction could be a crucial pathology in the increased TBI severity. By surgically removing the thyroid glands, which recapitulates the age-related increase in TBI-susceptible phenotypes, we provide direct evidence showing that endogenous thyroid hormone-dependent compensatory regulation of antioxidant events modulates individual TBI susceptibility, which is abolished in aged or thyroidectomized individuals. The antioxidant capacity of melatonin is well-known, and we found acute melatonin treatment but not liothyronine (T3) supplementation improved the TBI-susceptible phenotypes of oxidative stress, excitotoxic neuronal loss and promotes functional recovery in the aged individuals with thyroid dysfunction. Our study suggests that monitoring thyroid function and acute administration of melatonin could be feasible therapeutics in the management of geriatric-TBI in clinic.
Keywords: aging; melatonin; oxidative stress; thyroid dysfunction; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lo J., Chan L., Flynn S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021;102:115–131. doi: 10.1016/j.apmr.2020.04.001. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

