Transcriptomic Profiling and Pathway Analysis of Mesenchymal Stem Cells Following Low Dose-Rate Radiation Exposure
- PMID: 36829800
- PMCID: PMC9951969
- DOI: 10.3390/antiox12020241
Transcriptomic Profiling and Pathway Analysis of Mesenchymal Stem Cells Following Low Dose-Rate Radiation Exposure
Abstract
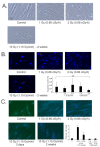
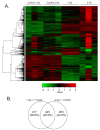
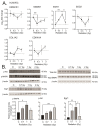
Low dose-rate radiation exposure can occur in medical imaging, as background from environmental or industrial radiation, and is a hazard of space travel. In contrast with high dose-rate radiation exposure that can induce acute life-threatening syndromes, chronic low-dose radiation is associated with Chronic Radiation Syndrome (CRS), which can alter environmental sensitivity. Secondary effects of chronic low dose-rate radiation exposure include circulatory, digestive, cardiovascular, and neurological diseases, as well as cancer. Here, we investigated 1-2 Gy, 0.66 cGy/h, 60Co radiation effects on primary human mesenchymal stem cells (hMSC). There was no significant induction of apoptosis or DNA damage, and cells continued to proliferate. Gene ontology (GO) analysis of transcriptome changes revealed alterations in pathways related to cellular metabolism (cholesterol, fatty acid, and glucose metabolism), extracellular matrix modification and cell adhesion/migration, and regulation of vasoconstriction and inflammation. Interestingly, there was increased hypoxia signaling and increased activation of pathways regulated by iron deficiency, but Nrf2 and related genes were reduced. The data were validated in hMSC and human lung microvascular endothelial cells using targeted qPCR and Western blotting. Notably absent in the GO analysis were alteration pathways for DNA damage response, cell cycle inhibition, senescence, and pro-inflammatory response that we previously observed for high dose-rate radiation exposure. Our findings suggest that cellular gene transcription response to low dose-rate ionizing radiation is fundamentally different compared to high-dose-rate exposure. We hypothesize that cellular response to hypoxia and iron deficiency are driving processes, upstream of the other pathway regulation.
Keywords: RNAseq; gene regulation; human microvascular endothelial cells; low dose-rate; mesenchymal stem cells; radiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources

