Common Reactivity and Properties of Heme Peroxidases: A DFT Study of Their Origin
- PMID: 36829861
- PMCID: PMC9952403
- DOI: 10.3390/antiox12020303
Common Reactivity and Properties of Heme Peroxidases: A DFT Study of Their Origin
Abstract
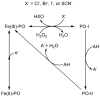
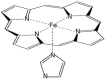
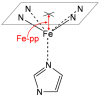
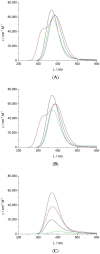
Electronic structure calculations using the density-functional theory (DFT) have been performed to analyse the effect of water molecules and protonation on the heme group of peroxidases in different redox (ferric, ferrous, compounds I and II) and spin states. Shared geometries, spectroscopic properties at the Soret region, and the thermodynamics of peroxidases are discussed. B3LYP and M06-2X density functionals with different basis sets were employed on a common molecular model of the active site (Fe-centred porphine and proximal imidazole). Computed Gibbs free energies indicate that the corresponding aquo complexes are not thermodynamically stable, supporting the five-coordinate Fe(III) centre in native ferric peroxidases, with a water molecule located at a non-bonding distance. Protonation of the ferryl oxygen of compound II is discussed in terms of thermodynamics, Fe-O bond distances, and redox properties. It is demonstrated that this protonation is necessary to account for the experimental data, and computed Gibbs free energies reveal pKa values of compound II about 8.5-9.0. Computation indicates that the general oxidative properties of peroxidase intermediates, as well as their reactivity towards water and protons and Soret bands, are mainly controlled by the iron porphyrin and its proximal histidine ligand.
Keywords: compound I; compound II; density functional calculations; ferryl oxygen; peroxidase; reduction potential.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ortiz de Montellano P.R. Cytochrome P-450: Structure, Mechanism, and Biochemistry. Plenum Press; New York, NY, USA: 1986.
-
- Everse J., Everse K.E., Grisham M.B. Peroxidases in Chemistry and Biology. CRC Press; Boca Raton, FL, USA: 1991.
-
- Harris D.L., Loew G.H. Identification of putative peroxide intermediates of peroxidases by electronic structure and spectra calculations. J. Am. Chem. Soc. 1996;118:10588–10594. doi: 10.1021/ja9617247. - DOI
-
- Loew G., Dupuis M. Structure of a model transient peroxide intermediate of peroxidases by ab initio methods. J. Am. Chem. Soc. 1996;118:10584–10587. doi: 10.1021/ja961723e. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

