Sulodexide Prevents Hyperglycemia-Induced Endothelial Dysfunction and Oxidative Stress in Porcine Retinal Arterioles
- PMID: 36829947
- PMCID: PMC9952154
- DOI: 10.3390/antiox12020388
Sulodexide Prevents Hyperglycemia-Induced Endothelial Dysfunction and Oxidative Stress in Porcine Retinal Arterioles
Abstract
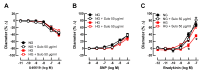
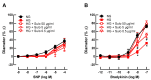
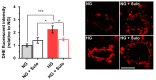
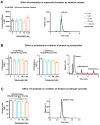
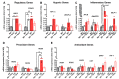
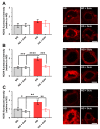
Diabetes mellitus may cause severe damage to retinal blood vessels. The central aim of this study was to test the hypothesis that sulodexide, a mixture of glycosaminoglycans, has a protective effect against hyperglycemia-induced endothelial dysfunction in the retina. Functional studies were performed in isolated porcine retinal arterioles. Vessels were cannulated and incubated with highly concentrated glucose solution (HG, 25 mM D-glucose) +/- sulodexide (50/5/0.5 μg/mL) or normally concentrated glucose solution (NG, 5.5 mM D-glucose) +/- sulodexide for two hours. Endothelium-dependent and endothelium-independent vasodilatation were measured by videomicroscopy. Reactive oxygen species (ROS) were quantified by dihydroethidium (DHE) fluorescence. Using high-pressure liquid chromatography (HPLC), the intrinsic antioxidant properties of sulodexide were investigated. Quantitative PCR was used to determine mRNA expression of regulatory, inflammatory, and redox genes in retinal arterioles, some of which were subsequently quantified at the protein level by immunofluorescence microscopy. Incubation of retinal arterioles with HG caused significant impairment of endothelium-dependent vasodilation, whereas endothelium-independent responses were not affected. In the HG group, ROS formation was markedly increased in the vascular wall. Strikingly, sulodexide had a protective effect against hyperglycemia-induced ROS formation in the vascular wall and had a concentration-dependent protective effect against endothelial dysfunction. Although sulodexide itself had only negligible antioxidant properties, it prevented hyperglycemia-induced overexpression of the pro-oxidant redox enzymes, NOX4 and NOX5. The data of the present study provide evidence that sulodexide has a protective effect against hyperglycemia-induced oxidative stress and endothelial dysfunction in porcine retinal arterioles, possibly by modulation of redox enzyme expression.
Keywords: diabetic retinopathy; endothelial dysfunction; oxidative stress; sulodexide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Responses of retinal arterioles and ciliary arteries in pigs with acute respiratory distress syndrome (ARDS).Exp Eye Res. 2019 Jul;184:152-161. doi: 10.1016/j.exer.2019.04.021. Epub 2019 Apr 22. Exp Eye Res. 2019. PMID: 31022399
-
Acute and Chronic Hyperglycemia Elicit JIP1/JNK-Mediated Endothelial Vasodilator Dysfunction of Retinal Arterioles.Invest Ophthalmol Vis Sci. 2016 Aug 1;57(10):4333-40. doi: 10.1167/iovs.16-19990. Invest Ophthalmol Vis Sci. 2016. PMID: 27556216 Free PMC article.
-
Sulodexide improves endothelial dysfunction in streptozotocin-induced diabetes in rats.Physiol Res. 2008;57(3):491-494. doi: 10.33549/physiolres.931506. Physiol Res. 2008. PMID: 18597586
-
Potential of Sulodexide in the Treatment of Diabetic Retinopathy and Retinal Vein Occlusion.Thromb Haemost. 2025 Apr;125(4):291-307. doi: 10.1055/s-0044-1791232. Epub 2024 Sep 18. Thromb Haemost. 2025. PMID: 39293483 Review.
-
Hypoxia and oxidative stress in the causation of diabetic retinopathy.Curr Diabetes Rev. 2011 Sep;7(5):291-304. doi: 10.2174/157339911797415620. Curr Diabetes Rev. 2011. PMID: 21916837 Review.
Cited by
-
Nox4 as a novel therapeutic target for diabetic vascular complications.Redox Biol. 2023 Aug;64:102781. doi: 10.1016/j.redox.2023.102781. Epub 2023 Jun 9. Redox Biol. 2023. PMID: 37321060 Free PMC article. Review.
-
Oxidative Stress: A Suitable Therapeutic Target for Optic Nerve Diseases?Antioxidants (Basel). 2023 Jul 20;12(7):1465. doi: 10.3390/antiox12071465. Antioxidants (Basel). 2023. PMID: 37508003 Free PMC article. Review.
-
Defenestration of Liver Sinusoidal Endothelial Cells: The Trigger of Liver Fibrosis.Pharmaceuticals (Basel). 2025 Jun 14;18(6):893. doi: 10.3390/ph18060893. Pharmaceuticals (Basel). 2025. PMID: 40573288 Free PMC article. Review.
-
Oxidative stress in the eye and its role in the pathophysiology of ocular diseases.Redox Biol. 2023 Dec;68:102967. doi: 10.1016/j.redox.2023.102967. Epub 2023 Nov 18. Redox Biol. 2023. PMID: 38006824 Free PMC article. Review.
-
Alleviate oxidative stress in diabetic retinopathy: antioxidant therapeutic strategies.Redox Rep. 2023 Dec;28(1):2272386. doi: 10.1080/13510002.2023.2272386. Epub 2023 Dec 2. Redox Rep. 2023. PMID: 38041593 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

