Influence of 2-Nitroimidazoles in the Response of FaDu Cells to Ionizing Radiation and Hypoxia/Reoxygenation Stress
- PMID: 36829948
- PMCID: PMC9951954
- DOI: 10.3390/antiox12020389
Influence of 2-Nitroimidazoles in the Response of FaDu Cells to Ionizing Radiation and Hypoxia/Reoxygenation Stress
Abstract
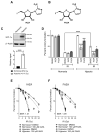
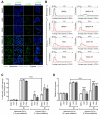
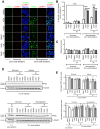
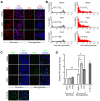
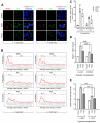
Cellular adaptations to hypoxia promote resistance to ionizing radiation (IR). This presents a challenge for treatment of head and neck cancer (HNC) that relies heavily on radiotherapy. Standard radiosensitizers often fail to reach diffusion-restricted hypoxic cells, whereas nitroimidazoles (NIs) [such as iodoazomycin arabinofuranoside (IAZA) and fluoroazomycin arabinofuranoside (FAZA)] can preferentially accumulate in hypoxic tumours. Here, we explored if the hypoxia-selective uptake of IAZA and FAZA could be harnessed to make HNC cells (FaDu) susceptible to radiation therapy. Cellular response to treatment was assessed through clonogenic survival assays and by monitoring DNA damage (immunofluorescence staining of DNA damage markers, γ-H2AX and p-53BP1, and by alkaline comet assay). The effects of reoxygenation were studied using the following assays: estimation of nucleoside incorporation to assess DNA synthesis rates, immunofluorescent imaging of chromatin-associated replication protein A as a marker of replication stress, and quantification of reactive oxygen species (ROS). Both IAZA and FAZA sensitized hypoxic HNC cells to IR, albeit the former is a better radiosensitizer. Radiosensitization by these compounds was restricted only to hypoxic cells, with no visible effects under normoxia. IAZA and FAZA impaired cellular adaptation to reoxygenation; high levels of ROS, reduced DNA synthesis capacity, and signs of replication stress were observed in reoxygenated cells. Overall, our data highlight the therapeutic potentials of IAZA and FAZA for targeting hypoxic HNC cells and provide rationale for future preclinical studies.
Keywords: DNA damage; head and neck cancer; hypoxia; nitroimidazole; radiosensitizer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Culliney B., Birhan A., Young A.V., Choi W., Shulimovich M., Blum R.H. Management of locally advanced or unresectable head and neck cancer. Oncology. 2008;22:1152. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

