Mitochondrial-Targeted Antioxidant MitoQ-Mediated Autophagy: A Novel Strategy for Precise Radiation Protection
- PMID: 36830013
- PMCID: PMC9952602
- DOI: 10.3390/antiox12020453
Mitochondrial-Targeted Antioxidant MitoQ-Mediated Autophagy: A Novel Strategy for Precise Radiation Protection
Abstract
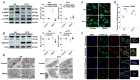
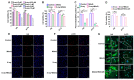
Radiotherapy (RT) is one of the most effective cancer treatments. However, successful radiation protection for normal tissue is a clinical challenge. Our previous study observed that MitoQ, a mitochondria-targeted antioxidant, was adsorbed to the inner mitochondrial membrane and remained the cationic moiety in the intermembrane space. The positive charges in MitoQ restrained the activity of respiratory chain complexes and decreased proton production. Therefore, a pseudo-mitochondrial membrane potential (PMMP) was developed via maintenance of exogenous positive charges. This study identified that PMMP constructed by MitoQ could effectively inhibit mitochondrial respiration within normal cells, disrupt energy metabolism, and activate adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK) signaling to induce autophagy. As such, it could not lead to starvation-induced autophagy among tumor cells due to the different energy phenotypes between normal and tumor cells (normal cells depend on mitochondrial respiration for energy supply, while tumor cells rely on aerobic glycolysis). Therefore, we successfully protected the normal cells from radiation-induced damage without affecting the tumor-killing efficacy of radiation by utilizing selective autophagy. MitoQ-constructed PMMP provides a new therapeutic strategy for specific radiation protection.
Keywords: MitoQ; PMMP; autophagy; energy phenotype; radioprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hamblin R., Vardon A., Akpalu J., Tampourlou M., Spiliotis I., Sbardella E., Lynch J., Shankaran V., Mavilakandy A., Gagliardi I., et al. Risk of second brain tumour after radiotherapy for pituitary adenoma or craniopharyngioma: A retrospective, multicentre, cohort study of 3679 patients with long-term imaging surveillance. Lancet Diabetes Endocrinol. 2022;10:581–588. doi: 10.1016/S2213-8587(22)00160-7. - DOI - PubMed
-
- Hong Z., Liu T., Wan L., Fa P., Kumar P., Cao Y., Prasad B., Qiu Z., Liu J., Wang H., et al. Targeting Squalene Epoxidase Interrupts Homologous Recombination via the ER Stress Response and Promotes Radiotherapy Efficacy. Cancer Res. 2022;82:1298–1312. doi: 10.1158/0008-5472.CAN-21-2229. - DOI - PMC - PubMed
-
- ClinicalTrials.gov: A Database of Privately and Publicly Funded Clinical Studies Conducted around the World. [(accessed on 13 December 2022)]; Available online: https://clinicaltrials.gov/ct2/results?cond=Radiation+Protection+and+can...
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

