Antioxidative and Cytoprotective Efficacy of Ethanolic Extracted Cranberry Pomace against Salmonella Enteritidis Infection in Chicken Liver Cells
- PMID: 36830018
- PMCID: PMC9952629
- DOI: 10.3390/antiox12020460
Antioxidative and Cytoprotective Efficacy of Ethanolic Extracted Cranberry Pomace against Salmonella Enteritidis Infection in Chicken Liver Cells
Abstract
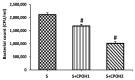
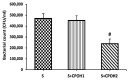
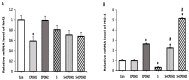
Salmonella enterica serovar Enteritidis is a globally significant zoonotic foodborne pathogen. Chicken liver is a vital organ that has been recently implicated in several reported human salmonellosis outbreaks in the U.S. One promising strategy for reducing Salmonella in chickens could be through supplementation with natural antimicrobial additives. Ethanolic extracted cranberry pomace (CPOH) is an excellent source of bioactive polyphenolic compounds with antioxidant and antimicrobial activities. However, the protective effect of CPOH against S. Enteritidis-induced chicken hepatic cell damage remains unclear. In this study, we used a chicken hepatoma cell (LMH) infection model to investigate the protective effects and potential mechanisms of CPOH. CPOH increased the viability of S. Enteritidis-infected LMH cells. Furthermore, CPOH reduced the adhesion and invasion of S. Enteritidis to LMH cells. CPOH downregulated the expression of Rho GTPase genes that are essential for Salmonella's entry into LMH cells. Additionally, the expression of antioxidant regulatory genes, such as Nrf2, HO-1, Txn, and Gclc, was increased. Our data show that CPOH effectively protected LMH cells from cell damage through the inhibition of S. Enteritidis adhesion and invasion, as well as the induction of the expression of master antioxidant genes. These findings offer opportunities to develop sustainable, safe, and economic strategies to reduce the colonization and pathogenesis of Salmonella.
Keywords: S. Enteritidis; anti-infection; antioxidant regulatory genes; cranberry pomace extract; cytoprotecting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- OECD/FAO . OECD-FAO Agricultural Outlook 2021–2030. OECD Publishing; Paris, France: 2021.
-
- El-Hack M.E.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B., Taha A.E., Soliman S.M., Ahmed A.E., El-Kott A.F., et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022;101:101696. doi: 10.1016/j.psj.2022.101696. - DOI - PMC - PubMed
-
- Hernández-Ramírez J.O., Nava-Ramírez M.J., Merino-Guzmán R., Téllez-Isaías G., Vázquez-Durán A., Méndez-Albores A. The effect of moderate-dose aflatoxin B1 and Salmonella Enteritidis infection on intestinal permeability in broiler chickens. Mycotoxin Res. 2019;36:31–39. doi: 10.1007/s12550-019-00367-7. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

