Oxidative Stress in Long-Term Exposure to Multi-Walled Carbon Nanotubes in Male Rats
- PMID: 36830022
- PMCID: PMC9952213
- DOI: 10.3390/antiox12020464
Oxidative Stress in Long-Term Exposure to Multi-Walled Carbon Nanotubes in Male Rats
Abstract
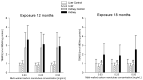
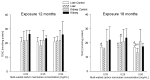
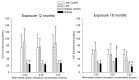
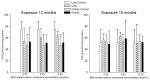
Multi-walled carbon nanotubes (MWCNTs) serve as nanoparticles due to their size, and for that reason, when in contact with the biological system, they can have toxic effects. One of the main mechanisms responsible for nanotoxicity is oxidative stress resulting from the production of intracellular reactive oxygen species (ROS). Therefore, oxidative stress biomarkers are important tools for assessing MWCNTs toxicity. The aim of this study was to evaluate the oxidative stress of multi-walled carbon nanotubes in male rats. Our animal model studies of MWCNTs (diameter ~15-30 nm, length ~15-20 μm) include measurement of oxidative stress parameters in the body fluid and tissues of animals after long-term exposure. Rattus Norvegicus/Wistar male rats were administrated a single injection to the knee joint at three concentrations: 0.03 mg/mL, 0.25 mg/mL, and 0.5 mg/mL. The rats were euthanized 12 and 18 months post-exposure by drawing blood from the heart, and their liver and kidney tissues were removed. To evaluate toxicity, the enzymatic activity of total protein (TP), reduced glutathione (GSH), glutathione S-transferase (GST), thiobarbituric acid reactive substances (TBARS), Trolox equivalent antioxidant capacity (TEAC), nitric oxide (NO), and catalase (CAT) was measured and histopathological examination was conducted. Results in rat livers showed that TEAC level was decreased in rats receiving nanotubes at higher concentrations. Results in kidneys report that the level of NO showed higher concentration after long exposure, and results in animal serums showed lower levels of GSH in rats exposed to nanotubes at higher concentrations. The 18-month exposure also resulted in a statistically significant increase in GST activity in the group of rats exposed to nanotubes at higher concentrations compared to animals receiving MWCNTs at lower concentrations and compared to the control group. Therefore, an analysis of oxidative stress parameters can be a key indicator of the toxic potential of multi-walled carbon nanotubes.
Keywords: long-term toxicity; multi-walled carbon nanotubes; oxidative stress parameters; rats.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Mauricio M.D., Guerra-Ojeda S., Marchio P., Valles S.L., Aldasoro M., Escribano-Lopez I., Herance J.R., Rocha M., Vila J.M., Victor V.M., et al. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxid. Med. Cell Longev. 2018;2018:6231482. doi: 10.1155/2018/6231482. - DOI - PMC - PubMed
-
- Powers K.W., Palazuelos M., Moudgil B.M., Roberts S.M. Characterization of the Size, Shape, and State of Dispersion of Nanoparticles for Toxicological Studies. Nanotoxicology. 2007;1:42–51. doi: 10.1080/17435390701314902. - DOI
-
- Caballero-Díaz E., Pfeiffer C., Kastl L., Rivera-Gil P., Simonet B., Valcárcel M., Jiménez-Lamana J., Laborda F., Parak W.J. The Toxicity of Silver Nanoparticles Depends on Their Uptake by Cells and Thus on Their Surface Chemistry. Part. Part. Syst. Charact. 2013;30:1079–1085. doi: 10.1002/ppsc.201300215. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

