Application of Plant Growth-Promoting Bacteria from Cape Verde to Increase Maize Tolerance to Salinity
- PMID: 36830045
- PMCID: PMC9952022
- DOI: 10.3390/antiox12020488
Application of Plant Growth-Promoting Bacteria from Cape Verde to Increase Maize Tolerance to Salinity
Abstract
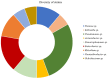
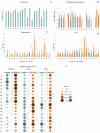
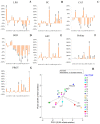
Salinity constitutes a major abiotic factor that negatively affects crop productivity. Inoculation with plant growth-promoting bacteria (PGPB) is proven to increase plant tolerance to abiotic stresses and enhance plant growth, development and productivity. The present study aims to increase the resilience of crops to salinity using bacteria from the microbiome of plants growing in saline environments. For that, the halotolerance of bacteria present in the roots of natural plants growing on Sal Island, which is characterized by its arid environment and maritime influence, was determined, with some strains having extreme halotolerance. Their ability to produce plant growth-promoting traits was evaluated, with most strains increasing indole acetic acid (26-418%), siderophore (>300%) and alginate (2-66%) production and phosphate solubilization (13-100%) under salt stress. The strains evidencing the best performance were inoculated in maize (Zea mays L.) plants and their influence on plant growth and biochemical status was evaluated. Results evidenced bacterial ability to especially increase proline (55-191%), whose osmotic, antioxidant and protein-protecting properties reduced protein damage in salt-stressed maize plants, evidencing the potential of PGPB to reduce the impact of salinity on crops. Enhanced nutrition, phytohormone production and osmolyte synthesis along with antioxidant response all contribute to increasing plant tolerance to salt stress.
Keywords: arid regions; maize; plant growth-promoting bacteria (PGPB); salinization; sustainability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Bioprospecting Soil Bacteria from Arid Zones to Increase Plant Tolerance to Drought: Growth and Biochemical Status of Maize Inoculated with Plant Growth-Promoting Bacteria Isolated from Sal Island, Cape Verde.Plants (Basel). 2022 Oct 29;11(21):2912. doi: 10.3390/plants11212912. Plants (Basel). 2022. PMID: 36365367 Free PMC article.
-
Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress.Microbiol Res. 2021 Jan;242:126616. doi: 10.1016/j.micres.2020.126616. Epub 2020 Oct 9. Microbiol Res. 2021. PMID: 33115624
-
Microbacterium azadirachtae CNUC13 Enhances Salt Tolerance in Maize by Modulating Osmotic and Oxidative Stress.Biology (Basel). 2024 Apr 7;13(4):244. doi: 10.3390/biology13040244. Biology (Basel). 2024. PMID: 38666856 Free PMC article.
-
Synergistic potential of halophytes and halophilic/halotolerant plant growth-promoting bacteria in saline soil remediation: Adaptive mechanisms, challenges, and sustainable solutions.Microbiol Res. 2025 Sep;298:128227. doi: 10.1016/j.micres.2025.128227. Epub 2025 May 22. Microbiol Res. 2025. PMID: 40440870 Review.
-
Salt-tolerant plant growth-promoting bacteria as a versatile tool for combating salt stress in crop plants.Arch Microbiol. 2024 Jul 5;206(8):341. doi: 10.1007/s00203-024-04071-8. Arch Microbiol. 2024. PMID: 38967784 Review.
Cited by
-
Inducing Drought Resilience in Maize Through Encapsulated Bacteria: Physiological and Biochemical Adaptations.Plants (Basel). 2025 Mar 5;14(5):812. doi: 10.3390/plants14050812. Plants (Basel). 2025. PMID: 40094834 Free PMC article.
-
Biodegradable Microplastics from Agricultural Mulch Films: Implications for Plant Growth-Promoting Bacteria and Plant's Oxidative Stress.Antioxidants (Basel). 2025 Feb 18;14(2):230. doi: 10.3390/antiox14020230. Antioxidants (Basel). 2025. PMID: 40002414 Free PMC article.
-
Parallel evolution of salinity tolerance in Arabidopsis thaliana accessions from Cape Verde Islands.Sci Adv. 2025 Jul 11;11(28):eadq8210. doi: 10.1126/sciadv.adq8210. Epub 2025 Jul 11. Sci Adv. 2025. PMID: 40644542 Free PMC article.
References
-
- Shahid S.A., Zaman M., Heng L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques. Springer; Cham, Switzerland: 2018. Soil Salinity: Historical Perspectives and a World Overview of the Problem; pp. 43–53. - DOI
-
- Akbari M., Alamdarlo H.N., Mosavi S.H. The Effects of Climate Change and Groundwater Salinity on Farmers’ Income Risk. Ecol. Indic. 2020;110:105893. doi: 10.1016/j.ecolind.2019.105893. - DOI
-
- Corwin D.L. Climate Change Impacts on Soil Salinity in Agricultural Areas. Eur. J. Soil Sci. 2021;72:842–862. doi: 10.1111/ejss.13010. - DOI
-
- Vengosh A. Salinization and Saline Environments. In: Lollar B.S., editor. Treatise on Geochemistry. Volume 9. Elsevier Inc.; Amsterdam, The Netherlands: 2003. pp. 333–365.
Grants and funding
LinkOut - more resources
Full Text Sources

