Itaconate Suppresses the Activation of Mitochondrial NLRP3 Inflammasome and Oxidative Stress in Allergic Airway Inflammation
- PMID: 36830047
- PMCID: PMC9951851
- DOI: 10.3390/antiox12020489
Itaconate Suppresses the Activation of Mitochondrial NLRP3 Inflammasome and Oxidative Stress in Allergic Airway Inflammation
Abstract
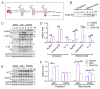
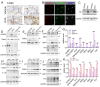
Itaconate has emerged as a novel anti-inflammatory and antioxidative endogenous metabolite, yet its role in allergic airway inflammation (AAI) and the underlying mechanism remains elusive. Here, the itaconate level in the lung was assessed by High Performance Liquid Chromatography (HPLC), and the effects of the Irg1/itaconate pathway on AAI and alveolar macrophage (AM) immune responses were evaluated using an ovalbumin (OVA)-induced AAI model established by wild type (WT) and Irg1-/- mice, while the mechanism of this process was investigated by metabolomics analysis, mitochondrial/cytosolic protein fractionation and transmission electron microscopy in the lung tissues. The results demonstrated that the Irg1 mRNA/protein expression and itaconate production in the lung were significantly induced by OVA. Itaconate ameliorated while Irg1 deficiency augmented AAI, and this may be attributed to the fact that itaconate suppressed mitochondrial events such as NLRP3 inflammasome activation, oxidative stress and metabolic dysfunction. Furthermore, we identified that the Irg1/itaconate pathway impacted the NLRP3 inflammasome activation and oxidative stress in AMs. Collectively, our findings provide evidence for the first time, supporting the conclusion that in the allergic lung, the itaconate level is markedly increased, which directly regulates AMs' immune responses. We therefore propose that the Irg1/itaconate pathway in AMs is a potential anti-inflammatory and anti-oxidative therapeutic target for AAI.
Keywords: Irg1; NLRP3 inflammasome; allergic airway inflammation; alveolar macrophage; itaconate; mitochondrial; oxidative stress.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- GINA Executive and Science Committee Global Strategy for Asthma Management and Prevention 2021. 2021. [(accessed on 3 May 2022)]. Available online: https://ginasthma.org/gina-reports/
-
- Michelucci A., Cordes T., Ghelfi J., Pailot A., Reiling N., Goldmann O., Binz T., Wegner A., Tallam A., Rausell A., et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. - DOI - PMC - PubMed
-
- Lampropoulou V., Sergushichev A., Bambouskova M., Nair S., Vincent E.E., Loginicheva E., Cervantes-Barragan L., Ma X., Huang S.C., Griss T., et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016;24:158–166. doi: 10.1016/j.cmet.2016.06.004. - DOI - PMC - PubMed
Grants and funding
- 81770032/National Natural Science Foundation of China
- 81870036/National Natural Science Foundation of China
- 1908085MH247/Natural Science Foundation of Anhui Province of China
- 2022AH051168/Natural Science Research Project of Anhui Educational Committee
- 2022xkj166/Foundation of Anhui Medical University
LinkOut - more resources
Full Text Sources

