CD38-Induced Metabolic Dysfunction Primes Multiple Myeloma Cells for NAD+-Lowering Agents
- PMID: 36830052
- PMCID: PMC9952390
- DOI: 10.3390/antiox12020494
CD38-Induced Metabolic Dysfunction Primes Multiple Myeloma Cells for NAD+-Lowering Agents
Abstract
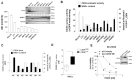
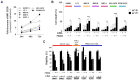
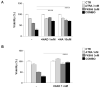
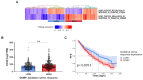
Cancer cells fuel growth and energy demands by increasing their NAD+ biosynthesis dependency, which therefore represents an exploitable vulnerability for anti-cancer strategies. CD38 is a NAD+-degrading enzyme that has become crucial for anti-MM therapies since anti-CD38 monoclonal antibodies represent the backbone for treatment of newly diagnosed and relapsed multiple myeloma patients. Nevertheless, further steps are needed to enable a full exploitation of these strategies, including deeper insights of the mechanisms by which CD38 promotes tumorigenesis and its metabolic additions that could be selectively targeted by therapeutic strategies. Here, we present evidence that CD38 upregulation produces a pervasive intracellular-NAD+ depletion, which impairs mitochondrial fitness and enhances oxidative stress; as result, genetic or pharmacologic approaches that aim to modify CD38 surface-level prime MM cells to NAD+-lowering agents. The molecular mechanism underlying this event is an alteration in mitochondrial dynamics, which decreases mitochondria efficiency and triggers energetic remodeling. Overall, we found that CD38 handling represents an innovative strategy to improve the outcomes of NAD+-lowering agents and provides the rationale for testing these very promising agents in clinical studies involving MM patients.
Keywords: NAD+ biosynthetic pathway; NAD+-lowering agents; cancer metabolism; mitochondrial disfunction; multiple myeloma; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Endocytosis-mediated mitochondrial transplantation: Transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity.Theranostics. 2019 May 26;9(12):3595-3607. doi: 10.7150/thno.33100. eCollection 2019. Theranostics. 2019. PMID: 31281500 Free PMC article.
-
CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats.Aging (Albany NY). 2020 Jun 7;12(12):11325-11336. doi: 10.18632/aging.103410. Epub 2020 Jun 7. Aging (Albany NY). 2020. PMID: 32507768 Free PMC article.
-
The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging.Trends Pharmacol Sci. 2018 Apr;39(4):424-436. doi: 10.1016/j.tips.2018.02.001. Epub 2018 Feb 23. Trends Pharmacol Sci. 2018. PMID: 29482842 Free PMC article. Review.
-
Targeting NAD+ Synthesis to Potentiate CD38-Based Immunotherapy of Multiple Myeloma.Trends Cancer. 2020 Jan;6(1):9-12. doi: 10.1016/j.trecan.2019.11.005. Epub 2019 Dec 31. Trends Cancer. 2020. PMID: 31952784
-
CD38 in Adenosinergic Pathways and Metabolic Re-programming in Human Multiple Myeloma Cells: In-tandem Insights From Basic Science to Therapy.Front Immunol. 2019 Apr 24;10:760. doi: 10.3389/fimmu.2019.00760. eCollection 2019. Front Immunol. 2019. PMID: 31068926 Free PMC article. Review.
Cited by
-
Clonal hematopoiesis impacts frailty in newly diagnosed multiple myeloma patients: a retrospective multicenter analysis.Sci Rep. 2024 Nov 26;14(1):29394. doi: 10.1038/s41598-024-79748-7. Sci Rep. 2024. PMID: 39592674 Free PMC article.
-
Impact of immune cell metabolism on membranous nephropathy and prospective therapy.Commun Biol. 2025 Mar 10;8(1):405. doi: 10.1038/s42003-025-07816-3. Commun Biol. 2025. PMID: 40065158 Free PMC article. Review.
-
Decoding NAD+ Metabolism in COVID-19: Implications for Immune Modulation and Therapy.Vaccines (Basel). 2024 Dec 24;13(1):1. doi: 10.3390/vaccines13010001. Vaccines (Basel). 2024. PMID: 39852780 Free PMC article. Review.
-
CD56 expression modulates NAD+ metabolic landscape and predicts sensitivity to anti-CD38 therapies in multiple myeloma.Blood Cancer J. 2025 May 2;15(1):83. doi: 10.1038/s41408-025-01284-y. Blood Cancer J. 2025. PMID: 40316562 Free PMC article. No abstract available.
-
Pathobiochemistry of Aging and Neurodegeneration: Deregulation of NAD+ Metabolism in Brain Cells.Biomolecules. 2024 Dec 6;14(12):1556. doi: 10.3390/biom14121556. Biomolecules. 2024. PMID: 39766263 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

