Selenium Protects Mouse Hypothalamic Cells from Glucocorticoid-Induced Endoplasmic Reticulum Stress Vulnerability and Insulin Signaling Impairment
- PMID: 36830084
- PMCID: PMC9952756
- DOI: 10.3390/antiox12020526
Selenium Protects Mouse Hypothalamic Cells from Glucocorticoid-Induced Endoplasmic Reticulum Stress Vulnerability and Insulin Signaling Impairment
Abstract
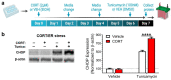
The use of glucocorticoid medications is known to cause metabolic side effects such as overeating, excess weight gain, and insulin resistance. The hypothalamus, a central regulator of feeding behavior and energy expenditure, is highly responsive to glucocorticoids, and it has been proposed that it plays a role in glucocorticoid-induced metabolic defects. Glucocorticoids can alter the expression and activity of antioxidant enzymes and promote the accumulation of reactive oxygen species. Recent evidence indicates that selenium can counter the effects of glucocorticoids, and selenium is critical for proper hypothalamic function. This study sought to determine whether selenium is capable of protecting hypothalamic cells from dysfunction caused by glucocorticoid exposure. We treated mHypoE-44 mouse hypothalamic cells with corticosterone to study the effects on cellular physiology and the involvement of selenium. We found that corticosterone administration rendered cells more vulnerable to endoplasmic reticulum stress and the subsequent impairment of insulin signaling. Supplementing the cell culture media with additional selenium alleviated endoplasmic reticulum stress and promoted insulin signaling. These findings implicate a protective role of selenium against chronic glucocorticoid-induced hypothalamic dysfunction.
Keywords: corticosterone; endoplasmic reticulum stress; glucocorticoid; hypothalamus; insulin; selenium.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Wray J.R., Davies A., Sefton C., Allen T.J., Adamson A., Chapman P., Lam B.Y.H., Yeo G.S.H., Coll A.P., Harno E., et al. Global transcriptomic analysis of the arcuate nucleus following chronic glucocorticoid treatment. Mol. Metab. 2019;26:5–17. doi: 10.1016/j.molmet.2019.05.008. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

