Co-Targeting of BTK and TrxR as a Therapeutic Approach to the Treatment of Lymphoma
- PMID: 36830087
- PMCID: PMC9952695
- DOI: 10.3390/antiox12020529
Co-Targeting of BTK and TrxR as a Therapeutic Approach to the Treatment of Lymphoma
Abstract
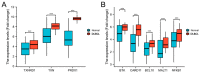
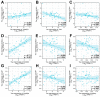
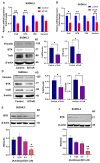
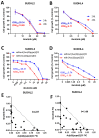
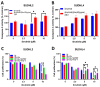
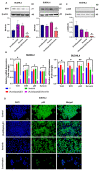
Diffuse large B-cell lymphoma (DLBCL) is a haematological malignancy representing the most diagnosed non-Hodgkin's lymphoma (NHL) subtype. Despite the approved chemotherapies available in clinics, some patients still suffer from side effects and relapsed disease. Recently, studies have reported the role of the Trx system and the BCR signalling pathway in cancer development and drug resistance. In this regard, we assessed a potential link between the two systems and evaluated the effects of [Au(d2pype)2]Cl (TrxR inhibitor) and ibrutinib (BTK inhibitor) alone and in combination on the cell growth of two DLBCL lymphoma cell lines, SUDHL2 and SUDHL4. In this study, we show higher expression levels of the Trx system and BCR signalling pathway in the DLBCL patient samples compared to the healthy samples. The knockdown of TrxR using siRNA reduced BTK mRNA and protein expression. A combination treatment with [Au(d2pype)2]Cl and ibrutinib had a synergistic effect on the inhibition of lymphoma cell proliferation, the activation of apoptosis, and, depending on lymphoma cell subtype, ferroptosis. Decreased BTK expression and the cytoplasmic accumulation of p65 were observed after the combination treatment in the DLBCL cells, indicating the inhibition of the NF-κB pathway. Thus, the co-targeting of BTK and TrxR may be an effective therapeutic strategy to consider for DLBCL treatment.
Keywords: B-cell receptor signaling pathway; DLBCL; bruton’s tyrosine kinase; thioredoxin reductase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Combination of Enzastaurin and Ibrutinib synergistically induces anti-tumor effects in diffuse large B cell lymphoma.J Exp Clin Cancer Res. 2019 Feb 18;38(1):86. doi: 10.1186/s13046-019-1076-4. J Exp Clin Cancer Res. 2019. PMID: 30777096 Free PMC article.
-
Investigating the Thioredoxin and Glutathione Systems' Response in Lymphoma Cells after Treatment with [Au(d2pype)2]CL.Antioxidants (Basel). 2021 Jan 13;10(1):104. doi: 10.3390/antiox10010104. Antioxidants (Basel). 2021. PMID: 33451071 Free PMC article.
-
The role of PIM1 in the ibrutinib-resistant ABC subtype of diffuse large B-cell lymphoma.Am J Cancer Res. 2016 Nov 1;6(11):2489-2501. eCollection 2016. Am J Cancer Res. 2016. PMID: 27904766 Free PMC article.
-
Targeting Bruton's tyrosine kinase with ibrutinib in B-cell malignancies.Clin Pharmacol Ther. 2015 May;97(5):455-68. doi: 10.1002/cpt.85. Epub 2015 Apr 3. Clin Pharmacol Ther. 2015. PMID: 25669675 Review.
-
Role of Bruton's tyrosine kinase in B cells and malignancies.Mol Cancer. 2018 Feb 19;17(1):57. doi: 10.1186/s12943-018-0779-z. Mol Cancer. 2018. PMID: 29455639 Free PMC article. Review.
Cited by
-
Regulation and therapy: the role of ferroptosis in DLBCL.Front Pharmacol. 2025 Jan 6;15:1458412. doi: 10.3389/fphar.2024.1458412. eCollection 2024. Front Pharmacol. 2025. PMID: 39834804 Free PMC article. Review.
-
Exploring the Role of Thioredoxin system in Cancer Immunotherapy.J Cancer. 2025 Jan 1;16(1):66-80. doi: 10.7150/jca.98306. eCollection 2025. J Cancer. 2025. PMID: 39744566 Free PMC article.
References
-
- Ichikawa A., Miyoshi H., Yamauchi T., Arakawa F., Kawano R., Muta H., Sugita Y., Akashi K., Ohshima K. Composite lymphoma of peripheral T-cell lymphoma and Hodgkin lymphoma, mixed cellularity type; pathological and molecular analysis. Pathol. Int. 2017;67:194–201. doi: 10.1111/pin.12515. - DOI - PubMed
LinkOut - more resources
Full Text Sources

