Development of a Clinical Score to Stratify the Risk for Carbapenem-Resistant Enterobacterales Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation
- PMID: 36830136
- PMCID: PMC9952392
- DOI: 10.3390/antibiotics12020226
Development of a Clinical Score to Stratify the Risk for Carbapenem-Resistant Enterobacterales Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation
Abstract
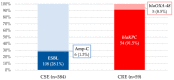
Identifying the risk factors for carbapenem-resistant Enterobacterales (CRE) bacteremia in cancer and hematopoietic stem cell transplantation (HSCT) patients would allow earlier initiation of an appropriate empirical antibiotic treatment. This is a prospective multicenter observational study in patients from 12 centers in Argentina, who presented with cancer or hematopoietic stem-cell transplant and developed Enterobacterales bacteremia. A multiple logistic regression model identified risk factors for CRE bacteremia, and a score was developed according to the regression coefficient. This was validated by the bootstrap resampling technique. Four hundred and forty-three patients with Enterobacterales bacteremia were included: 59 with CRE and 384 with carbapenem-susceptible Enterobacterales (CSE). The risk factors that were identified and the points assigned to each of them were: ≥10 days of hospitalization until bacteremia: OR 4.03, 95% CI 1.88-8.66 (2 points); previous antibiotics > 7 days: OR 4.65, 95% CI 2.29-9.46 (2 points); current colonization with KPC-carbapenemase-producing Enterobacterales: 33.08, 95% CI 11.74-93.25 (5 points). With a cut-off of 7 points, a sensitivity of 35.59%, specificity of 98.43%, PPV of 77.7%, and NPV of 90.9% were obtained. The overall performance of the score was satisfactory (AUROC of 0.85, 95% CI 0.80-0.91). Finally, the post-test probability of CRE occurrence in patients with none of the risk factors was 1.9%, which would virtually rule out the presence of CRE bacteremia.
Keywords: Enterobacterales; bacteremia; cancer; score.
Conflict of interest statement
Fabián Herrera received speaker honoraria from MSD and Pfizer and a research and educational grant from Pfizer. Fernando Pasterán received speaker honoraria from MSD and Pfizer. Sandra Lambert received speaker honoraria from Pfizer. The rest of the authors declare no conflicts of interest.
Figures

References
-
- Capone A., Giannella M., Fortini D., Giordano A., Meledandri M., Ballardini M., Venditti M., Bordi E., Capozzi D., Balice M.P., et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin. Microbiol. Infect. 2013;19:E23–E30. doi: 10.1111/1469-0691.12070. - DOI - PubMed
-
- Castanheira M., Deshpande L.M., Mendes R.E., Canton R., Sader H.S., Jones R.N. Variations in the Occurrence of Resistance Phenotypes and Carbapenemase Genes Among Enterobacteriaceae Isolates in 20 Years of the SENTRY Antimicrobial Surveillance Program. Open Forum Infect. Dis. 2019;6((Suppl. S1)):S23–S33. doi: 10.1093/ofid/ofy347. - DOI - PMC - PubMed
-
- García-Betancur J.C., Appel T.M., Esparza G., Gales A.C., Levy-Hara G., Cornistein W., Vega S., Nuñez D., Cuellar L., Bavestrello L., et al. Update on the epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev. Anti-Infect. Ther. 2021;19:197–213. doi: 10.1080/14787210.2020.1813023. - DOI - PubMed
-
- Thomas G.R., Corso A., Pasterán F., Shal J., Sosa A., Pillonetto M., de Souza Peral R.T., Hormazábal J.C., Araya P., Saavedra S.Y., et al. Increased Detection of Carbapenemase-Producing Enterobacterales Bacteria in Latin America and the Caribbean during the COVID-19 Pandemic. Emerg. Infect. Dis. 2022;28:1–8. doi: 10.3201/eid2811.220415. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

