Antimicrobial Stewardship Program for Patients in the Hematological Department Receiving Carbapenem Therapy: A Single-Center and Interrupted Time Series Analysis
- PMID: 36830213
- PMCID: PMC9951935
- DOI: 10.3390/antibiotics12020302
Antimicrobial Stewardship Program for Patients in the Hematological Department Receiving Carbapenem Therapy: A Single-Center and Interrupted Time Series Analysis
Abstract
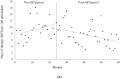
As antibiotic resistance has become a global problem, the intervention of an antimicrobial stewardship team (AST) is warranted. In hematological disorders, infectious complications are crucial owing to abnormal neutrophil function and decreased cell-mediated immunity. Despite the widespread implementation of AST intervention, the effectiveness of stewardship practices for immunocompromised patients remains uncertain. We determined the effect of AST interventions on carbapenem therapy in the department of hematology. Patients admitted to the department and undergoing carbapenem therapy were enrolled. We compared carbapenem use between the pre-AST (April 2016-March 2018) and post-AST (April 2018-March 2021) periods. Factors associated with long-term carbapenem therapy were investigated. Overall, 515 episodes of carbapenem therapy in 264 patients in the department were evaluated. According to the interrupted time series analysis, the number of days of therapy decreased with AST intervention (β = -0.263, p = 0.011). In multivariate analysis, predictive factors associated with long-term carbapenem therapy (>8 days) were outpatient onset, chronic obstructive pulmonary disease, acute myeloid leukemia, multiple myeloma, and infection with resistant bacteria (such as extended spectrum β-lactamases and AmpC) (95% confidence interval, 1.030-2.818, 1.067-66.667, 1.057-2.782, 0.168-0.742, and 1.382-5.750, respectively). The AST intervention reduced carbapenem use in patients with hematological disorders.
Keywords: antimicrobial stewardship; carbapenem; hematological disorders; interrupted time series analysis; predictive factors.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures


References
-
- Freifeld A.G., Bow E.J., Sepkowitz K.A., Boeckh M.J., Ito J.I., Mullen C.A., Raad I.I., Rolston K.V., Young J.H., Wingard J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. - DOI - PubMed
-
- Averbuch D., Orasch C., Cordonnier C., Livermore D.M., Mikulska M., Viscoli C., Gyssens I.C., Kern W.V., Klyasova G., Marchetti O., et al. ECIL4, a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID and ELN. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011. Haematol. 4th Eur Conf. Infect. Leuk. 2013;98:1826–1835. doi: 10.3324/haematol.2013.091025. - DOI - PMC - PubMed
-
- Link H., Böhme A., Cornely O.A., Höffken K., Kellner O., Kern W.V., Mahlberg R., Maschmeyer G., Nowrousian M.R., Ostermann H., et al. Antimicrobial therapy of unexplained fever in neutropenic patients—Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Study Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG-German Cancer Society) Ann. Hematol. 2003;82((Suppl. 2)):S105–S117. doi: 10.1007/s00277-003-0764-4. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

