Repurposing β-Lactams for the Treatment of Mycobacterium kansasii Infections: An In Vitro Study
- PMID: 36830246
- PMCID: PMC9952313
- DOI: 10.3390/antibiotics12020335
Repurposing β-Lactams for the Treatment of Mycobacterium kansasii Infections: An In Vitro Study
Abstract
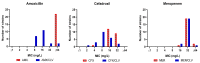
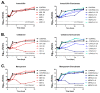
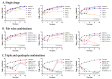
Mycobacterium kansasii (Mkn) causes tuberculosis-like lung infection in both immunocompetent and immunocompromised patients. Current standard therapy against Mkn infection is lengthy and difficult to adhere to. Although β-lactams are the most important class of antibiotics, representing 65% of the global antibiotic market, they have been traditionally dismissed for the treatment of mycobacterial infections, as they were considered inactive against mycobacteria. A renewed interest in β-lactams as antimycobacterial agents has shown their activity against several mycobacterial species, including M. tuberculosis, M. ulcerans or M. abscessus; however, information against Mkn is lacking. In this study, we determined the in vitro activity of several β-lactams against Mkn. A selection of 32 agents including all β-lactam chemical classes (penicillins, cephalosporins, carbapenems and monobactams) with three β-lactamase inhibitors (clavulanate, tazobactam and avibactam) were evaluated against 22 Mkn strains by MIC assays. Penicillins plus clavulanate and first- and third-generation cephalosporins were the most active β-lactams against Mkn. Combinatorial time-kill assays revealed favorable interactions of amoxicillin-clavulanate and cefadroxil with first-line Mkn treatment. Amoxicillin-clavulanate and cefadroxil are oral medications that are readily available, and well tolerated with an excellent safety and pharmacokinetic profile that could constitute a promising alternative option for Mkn therapy.
Keywords: Mycobacterium kansasii; amoxicillin–clavulanate; cefadroxil; nontuberculous mycobacteria; repurposing; β-lactam combinations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synergistic oral beta-lactam combinations for treating tuberculosis.J Appl Microbiol. 2024 Oct 3;135(10):lxae255. doi: 10.1093/jambio/lxae255. J Appl Microbiol. 2024. PMID: 39394664
-
In Vitro Activity of the New β-Lactamase Inhibitors Relebactam and Vaborbactam in Combination with β-Lactams against Mycobacterium abscessus Complex Clinical Isolates.Antimicrob Agents Chemother. 2019 Feb 26;63(3):e02623-18. doi: 10.1128/AAC.02623-18. Print 2019 Mar. Antimicrob Agents Chemother. 2019. PMID: 30642943 Free PMC article.
-
Strongly Bactericidal All-Oral β-Lactam Combinations for the Treatment of Mycobacterium abscessus Lung Disease.Antimicrob Agents Chemother. 2022 Sep 20;66(9):e0079022. doi: 10.1128/aac.00790-22. Epub 2022 Sep 1. Antimicrob Agents Chemother. 2022. PMID: 36047786 Free PMC article.
-
Dual β-lactam therapy to improve treatment outcome in Mycobacterium abscessus disease.Clin Microbiol Infect. 2024 Jun;30(6):738-742. doi: 10.1016/j.cmi.2024.03.019. Epub 2024 Mar 23. Clin Microbiol Infect. 2024. PMID: 38527611 Review.
-
Beta-lactam antibiotics: newer formulations and newer agents.Infect Dis Clin North Am. 2004 Sep;18(3):603-19, ix. doi: 10.1016/j.idc.2004.04.006. Infect Dis Clin North Am. 2004. PMID: 15308278 Review.
Cited by
-
Tackling Nontuberculous Mycobacteria by Repurposable Drugs and Potential Leads from Natural Products.Curr Top Med Chem. 2024;24(15):1291-1326. doi: 10.2174/0115680266276938240108060247. Curr Top Med Chem. 2024. PMID: 38288807 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

