Real-World Clinical Outcomes of Molnupiravir for the Treatment of Mild to Moderate COVID-19 in Adult Patients during the Dominance of the Omicron Variant: A Meta-Analysis
- PMID: 36830302
- PMCID: PMC9952148
- DOI: 10.3390/antibiotics12020393
Real-World Clinical Outcomes of Molnupiravir for the Treatment of Mild to Moderate COVID-19 in Adult Patients during the Dominance of the Omicron Variant: A Meta-Analysis
Abstract
Introduction: The therapeutic impact of molnupiravir in the Omicron variant phase is unknown. The goal of the current meta-analysis was to compare the real-world clinical outcomes of molnupiravir for the treatment of mild to moderate COVID-19 during the dominance of the Omicron variant in adult patients to that of a placebo.
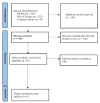
Methods: To be included, studies had to directly compare the clinical effectiveness of molnupiravir in treating adult COVID-19 patients to that of a placebo. Studies were included based on the following outcomes: all-cause mortality, composite outcome of disease progression, hospitalization rate, and viral load.
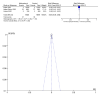
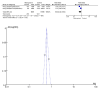
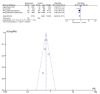
Results: The current meta-analysis included six studies that indicated that the risk of mortality was reduced by 34%, and the risk of composite outcome of disease progression was reduced by 37% among patients who received molnupiravir. Molnupiravir was associated with faster reduction in viral loads than the placebo. There was no clinical benefit of reducing all-cause mortality in mild to moderate COVID-19 patients with high COVID-19 vaccination coverage.
Conclusion: The clinical effectiveness of molnupiravir was associated with COVID-19 vaccination coverage in COVID-19 patients. There is a lack of detailed data on its effectiveness in vaccinated patients, especially those with low COVID-19 vaccination coverage.
Keywords: COVID-19; COVID-19 vaccination coverage; all-cause mortality; composite outcome of disease progression; molnupiravir; viral load.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Efficacy of Molnupiravir for the Treatment of Mild or Moderate COVID-19 in Adults: A Meta-Analysis.Cureus. 2023 May 5;15(5):e38586. doi: 10.7759/cureus.38586. eCollection 2023 May. Cureus. 2023. PMID: 37284377 Free PMC article. Review.
-
Molnupiravir Use Among Patients with COVID-19 in Real-World Settings: A Systematic Literature Review.Infect Dis Ther. 2024 Jun;13(6):1177-1198. doi: 10.1007/s40121-024-00976-5. Epub 2024 May 14. Infect Dis Ther. 2024. PMID: 38743192 Free PMC article. Review.
-
Real-world clinical outcomes of treatment with molnupiravir for patients with mild-to-moderate coronavirus disease 2019 during the Omicron variant pandemic.Clin Exp Med. 2023 Oct;23(6):2715-2723. doi: 10.1007/s10238-022-00949-3. Epub 2022 Dec 5. Clin Exp Med. 2023. PMID: 36469171 Free PMC article.
-
Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study.Lancet Infect Dis. 2022 Dec;22(12):1681-1693. doi: 10.1016/S1473-3099(22)00507-2. Epub 2022 Aug 24. Lancet Infect Dis. 2022. PMID: 36029795 Free PMC article.
-
Molnupiravir, Nirmatrelvir/Ritonavir, or Sotrovimab for High-Risk COVID-19 Patients Infected by the Omicron Variant: Hospitalization, Mortality, and Time until Negative Swab Test in Real Life.Pharmaceuticals (Basel). 2023 May 9;16(5):721. doi: 10.3390/ph16050721. Pharmaceuticals (Basel). 2023. PMID: 37242504 Free PMC article.
Cited by
-
Development and Validation of a High-Performance Liquid Chromatography with Tandem Mass Spectrometry (HPLC-MS/MS) Method for Quantification of Major Molnupiravir Metabolite (β-D-N4-hydroxycytidine) in Human Plasma.Biomedicines. 2023 Aug 23;11(9):2356. doi: 10.3390/biomedicines11092356. Biomedicines. 2023. PMID: 37760797 Free PMC article.
-
Efficacy of Molnupiravir for the Treatment of Mild or Moderate COVID-19 in Adults: A Meta-Analysis.Cureus. 2023 May 5;15(5):e38586. doi: 10.7759/cureus.38586. eCollection 2023 May. Cureus. 2023. PMID: 37284377 Free PMC article. Review.
-
Pathophysiology and clinical management of coronavirus disease (COVID-19): a mini-review.Front Immunol. 2023 Aug 14;14:1116131. doi: 10.3389/fimmu.2023.1116131. eCollection 2023. Front Immunol. 2023. PMID: 37646038 Free PMC article. Review.
-
Efficacy and Safety of Molnupiravir Treatment for COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Int J Antimicrob Agents. 2023 Aug;62(2):106870. doi: 10.1016/j.ijantimicag.2023.106870. Epub 2023 May 26. Int J Antimicrob Agents. 2023. PMID: 37245600 Free PMC article.
-
Microfluidic strategies for biomimetic lung chip establishment and SARS-CoV2 study.Mater Today Bio. 2023 Dec 7;24:100905. doi: 10.1016/j.mtbio.2023.100905. eCollection 2024 Feb. Mater Today Bio. 2023. PMID: 38094656 Free PMC article. Review.
References
-
- National Institutes of Health Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [(accessed on 17 February 2022)];2022 Available online: https://www.covid19treatmentguidelines.nih.gov. - PubMed
-
- US Food and Drug Administration Fact Sheet for Healthcare Providers: Emergency Use Authorization for Molnupiravir. [(accessed on 15 January 2022)]; Available online: https://www.fda.gov/media/155054/download.
-
- US Food and Drug Administration. [(accessed on 17 July 2022)]; Available online: https://www.fda.gov/media/155241/download.
-
- Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., Baniecki M., Hendrick V.M., Damle B., Simón-Campos A., et al. EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

