Yeast Cell Wall Compounds on The Formation of Fermentation Products and Fecal Microbiota in Cats: An In Vivo and In Vitro Approach
- PMID: 36830424
- PMCID: PMC9951743
- DOI: 10.3390/ani13040637
Yeast Cell Wall Compounds on The Formation of Fermentation Products and Fecal Microbiota in Cats: An In Vivo and In Vitro Approach
Abstract
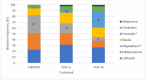
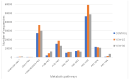
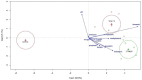
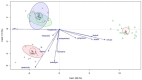
The effects of yeast cell wall compounds (YCWs) being added to cat food on hindgut fermentation metabolites and fecal microbiota were assessed in in vivo Experiment 1 (Exp. 1) and in vitro Experiments 2 and 3 (Exp. 2 and 3). In Exp. 1, the cats' diets were supplemented with two dietary concentrations (46.2 and 92.4 ppm) of YCWs (YCW-15 and YCW-30, respectively), and a negative control diet with no compound in three groups (six cats per group) was used to assess the fecal score, pH, digestibility, fermentation products, and microbiota. In Exp. 2, feces from the cats that were not supplemented with YCWs (control) were used as an inoculum. A blend of pectin, amino acids, and cellulose was used as a substrate, and the YCW compound was added at two levels (5 and 10 mg). In Exp. 3, feces from cats fed YCWs were used as an inoculum to test three different substrates (pectin, amino acids, and cellulose). In Exp. 2 and 3, the gas production, pH, and fermentation products (ammonia, SCFAs, and BCFAs) were assessed. YCW-30 resulted in a higher digestibility coefficient of the crude protein, organic matter (OM) (p < 0.05), and energy of the diet (p < 0.10). Regarding the fermentation products, YCW-15 showed a trend toward higher concentrations of propionate, acetate, lactate, ammonia, isobutyrate, and valerate, while YCW-30 showed a trend (p < 0.10) toward higher levels of butyrate and pH values. The bacteroidia class and the genus Prevotella were increased by using YCW-30 and the control. At the gender level, decreased (p < 0.01) Megasphaera was observed with YCW inclusion. The microbiota differed (p < 0.01) among the groups in their Shannon indexes. For beta diversity, YCW-30 showed higher indexes (p = 0.008) than the control. The microbiota metabolic profile differed in the pathway CENTFERM-PWY; it was more expressed in YCW-30 compared to the control. In Exp. 2, the YCWs showed a higher ratio (p = 0.006) of the fermentation products in the treatments with additives with a trend towards a high dose of the additive (10 mg). In Exp. 3, the effects of the substrates (p < 0.001), but not of the YCWs, on the fermentation products were observed, perhaps due to the low dietary concentrations we used. However, the marked responses of the fermentation products to the substrates validated the methodology. We could conclude that the YCWs, even at low dietary concentrations, affected fecal SCFA production, reduced the fecal pH, and modulated the fecal microbiota in the cats. These responses were more pronounced under in vitro conditions.
Keywords: beta-glucan; butyrate; feline; lactate; mannan oligosaccharide; microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Dos Santos Felssner K., Todesco H., Grande P.A., Ogoshi R.C.S., Dos Reis J.S., De Oliveira Borges Saad F.M., Vasconcellos R.S. Dietetic combination of mannan-oligosaccharides and fructooligosaccharides modifies nitrogen metabolism in dogs. Semin. Agrar. 2016;37:3335–3347. doi: 10.5433/1679-0359.2016v37n5p3335. - DOI
-
- Garcia-Mazcorro J.F., Minamoto Y. Gastrointestinal microorganisms in cats and dogs: A brief review. Arch. Med. Vet. 2013;45:111–124. doi: 10.4067/S0301-732X2013000200002. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

