Copper Binding and Redox Activity of α-Synuclein in Membrane-Like Environment
- PMID: 36830656
- PMCID: PMC9953312
- DOI: 10.3390/biom13020287
Copper Binding and Redox Activity of α-Synuclein in Membrane-Like Environment
Abstract
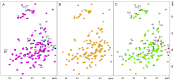
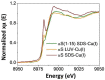
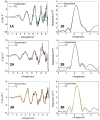
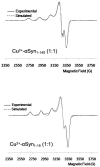
α-Synuclein (αSyn) constitutes the main protein component of Lewy bodies, which are the pathologic hallmark in Parkinson's disease. αSyn is unstructured in solution but the interaction of αSyn with lipid membrane modulates its conformation by inducing an α-helical structure of the N-terminal region. In addition, the interaction with metal ions can trigger αSyn conformation upon binding and/or through the metal-promoted generation of reactive oxygen species which lead to a cascade of structural alterations. For these reasons, the ternary interaction between αSyn, copper, and membranes needs to be elucidated in detail. Here, we investigated the structural properties of copper-αSyn binding through NMR, EPR, and XAS analyses, with particular emphasis on copper(I) coordination since the reduced state is particularly relevant for oxygen activation chemistry. The analysis was performed in different membrane model systems, such as micellar sodium dodecyl sulfate (SDS) and unilamellar vesicles, comparing the binding of full-length αSyn and N-terminal peptide fragments. The presence of membrane-like environments induced the formation of a copper:αSyn = 1:2 complex where Cu+ was bound to the Met1 and Met5 residues of two helical peptide chains. In this coordination, Cu+ is stabilized and is unreactive in the presence of O2 in catechol substrate oxidation.
Keywords: copper(I); copper(II); membrane environment; redox activity; synuclein; α-helix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

