Comparative Research: Regulatory Mechanisms of Ribosomal Gene Transcription in Saccharomyces cerevisiae and Schizosaccharomyces pombe
- PMID: 36830657
- PMCID: PMC9952952
- DOI: 10.3390/biom13020288
Comparative Research: Regulatory Mechanisms of Ribosomal Gene Transcription in Saccharomyces cerevisiae and Schizosaccharomyces pombe
Abstract
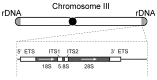
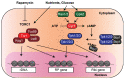
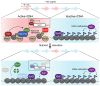
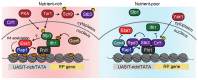
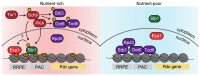
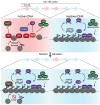
Restricting ribosome biosynthesis and assembly in response to nutrient starvation is a universal phenomenon that enables cells to survive with limited intracellular resources. When cells experience starvation, nutrient signaling pathways, such as the target of rapamycin (TOR) and protein kinase A (PKA), become quiescent, leading to several transcription factors and histone modification enzymes cooperatively and rapidly repressing ribosomal genes. Fission yeast has factors for heterochromatin formation similar to mammalian cells, such as H3K9 methyltransferase and HP1 protein, which are absent in budding yeast. However, limited studies on heterochromatinization in ribosomal genes have been conducted on fission yeast. Herein, we shed light on and compare the regulatory mechanisms of ribosomal gene transcription in two species with the latest insights.
Keywords: TOR pathway; TORC1; budding yeast; epigenetics; fission yeast; gene regulation; heterochromatin; ribosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
TOR inactivation triggers heterochromatin formation in rDNA during glucose starvation.Cell Rep. 2023 Nov 28;42(11):113320. doi: 10.1016/j.celrep.2023.113320. Epub 2023 Oct 31. Cell Rep. 2023. PMID: 37913773
-
The essential function of Rrs1 in ribosome biogenesis is conserved in budding and fission yeasts.Yeast. 2015 Sep;32(9):607-14. doi: 10.1002/yea.3083. Epub 2015 Jul 20. Yeast. 2015. PMID: 26122634
-
TOR and MAP kinase pathways synergistically regulate autophagy in response to nutrient depletion in fission yeast.Autophagy. 2022 Feb;18(2):375-390. doi: 10.1080/15548627.2021.1935522. Epub 2021 Jun 23. Autophagy. 2022. PMID: 34157946 Free PMC article.
-
The regional sequestration of heterochromatin structural proteins is critical to form and maintain silent chromatin.Epigenetics Chromatin. 2022 Jan 31;15(1):5. doi: 10.1186/s13072-022-00435-w. Epigenetics Chromatin. 2022. PMID: 35101096 Free PMC article. Review.
-
Novel Links between TORC1 and Traditional Non-Coding RNA, tRNA.Genes (Basel). 2020 Aug 19;11(9):956. doi: 10.3390/genes11090956. Genes (Basel). 2020. PMID: 32825021 Free PMC article. Review.
Cited by
-
The Product of the Fission Yeast fhl1 Gene Binds to the HomolE Box and Activates In Vitro Transcription of Ribosomal Protein Genes.Int J Mol Sci. 2023 May 30;24(11):9472. doi: 10.3390/ijms24119472. Int J Mol Sci. 2023. PMID: 37298423 Free PMC article.
-
Quitting Your Day Job in Response to Stress: Cell Survival and Cell Death Require Secondary Cytoplasmic Roles of Cyclin C and Med13.Cells. 2025 Apr 25;14(9):636. doi: 10.3390/cells14090636. Cells. 2025. PMID: 40358161 Free PMC article. Review.
-
Fission Yeast TORC1 Promotes Cell Proliferation through Sfp1, a Transcription Factor Involved in Ribosome Biogenesis.Mol Cell Biol. 2023;43(12):675-692. doi: 10.1080/10985549.2023.2282349. Epub 2023 Dec 20. Mol Cell Biol. 2023. PMID: 38051102 Free PMC article.
-
The CDK8 kinase module: A novel player in the transcription of translation initiation and ribosomal genes.Mol Biol Cell. 2025 Jan 1;36(1):ar2. doi: 10.1091/mbc.E24-04-0164. Epub 2024 Nov 20. Mol Biol Cell. 2025. PMID: 39565680 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

