Time- and Sex-Dependent Effects of Fingolimod Treatment in a Mouse Model of Alzheimer's Disease
- PMID: 36830699
- PMCID: PMC9953119
- DOI: 10.3390/biom13020331
Time- and Sex-Dependent Effects of Fingolimod Treatment in a Mouse Model of Alzheimer's Disease
Abstract
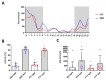
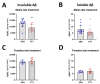
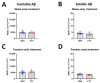
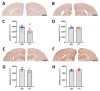
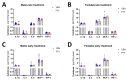
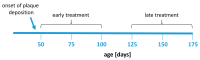
Alzheimer's disease (AD) is the most common cause of dementia. Fingolimod has previously shown beneficial effects in different animal models of AD. However, it has shown contradictory effects when it has been applied at early disease stages. Our objective was to evaluate fingolimod in two different treatment paradigms. To address this aim, we treated male and female APP-transgenic mice for 50 days, starting either before plaque deposition at 50 days of age (early) or at 125 days of age (late). To evaluate the effects, we investigated the neuroinflammatory and glial markers, the Aβ load, and the concentration of the brain-derived neurotrophic factor (BDNF). We found a reduced Aβ load only in male animals in the late treatment paradigm. These animals also showed reduced microglia activation and reduced IL-1β. No other treatment group showed any difference in comparison to the controls. On the other hand, we detected a linear correlation between BDNF and the brain Aβ concentrations. The fingolimod treatment has shown beneficial effects in AD models, but the outcome depends on the neuroinflammatory state at the start of the treatment. Thus, according to our data, a fingolimod treatment would be effective after the onset of the first AD symptoms, mainly affecting the neuroinflammatory reaction to the ongoing Aβ deposition.
Keywords: APPPS1; Alzheimer’s disease; FTY720; Gilenya; amyloid beta; fingolimod; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Alzheimer Association Report 2020 Alzheimer’s disease facts and figures. J. Alzheimers Dement. 2020;16:391–460. doi: 10.1002/alz.12068. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 263024513/Deutsche Forschungsgemeinschaft
- 2019054/Southern and Eastern Norway Regional Health Authority
- 19008/Barnekreftforeningen
- TO01000078/Technology Agency of the Czech Republic
- 295910, 327571/The Research Council of Norway
- ES RTD/2020/26/Latvian Council of Science
- 01ED2106/Federal Ministry of Education and Research
- 882717/Austrian Research Promotion Agency
- 8F21002/Ministry of Education Youth and Sports
- 20-JPW2-0002-04/Agence Nationale de la Recherche
- 2020-02905/Swedish Research Council
- 2019055/HelseSØ (Norway
- 2022046/HelseSØ (Norway
- 327571/Norges forskningsråd
- 295910/Norges forskningsråd
LinkOut - more resources
Full Text Sources
Medical

