Novel Green Fluorescent Polyamines to Analyze ATP13A2 and ATP13A3 Activity in the Mammalian Polyamine Transport System
- PMID: 36830711
- PMCID: PMC9953717
- DOI: 10.3390/biom13020337
Novel Green Fluorescent Polyamines to Analyze ATP13A2 and ATP13A3 Activity in the Mammalian Polyamine Transport System
Abstract
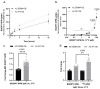
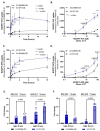
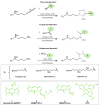
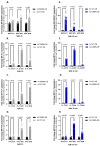
Cells acquire polyamines putrescine (PUT), spermidine (SPD) and spermine (SPM) via the complementary actions of polyamine uptake and synthesis pathways. The endosomal P5B-type ATPases ATP13A2 and ATP13A3 emerge as major determinants of mammalian polyamine uptake. Our biochemical evidence shows that fluorescently labeled polyamines are genuine substrates of ATP13A2. They can be used to measure polyamine uptake in ATP13A2- and ATP13A3-dependent cell models resembling radiolabeled polyamine uptake. We further report that ATP13A3 enables faster and stronger cellular polyamine uptake than does ATP13A2. We also compared the uptake of new green fluorescent PUT, SPD and SPM analogs using different coupling strategies (amide, triazole or isothiocyanate) and fluorophores (symmetrical BODIPY, BODIPY-FL and FITC). ATP13A2 promotes the uptake of various SPD and SPM analogs, whereas ATP13A3 mainly stimulates the uptake of PUT and SPD conjugates. However, the polyamine linker and coupling position on the fluorophore impacts the transport capacity, whereas replacing the fluorophore affects polyamine selectivity. The highest uptake in ATP13A2 or ATP13A3 cells is observed with BODIPY-FL-amide conjugated to SPD, whereas BODIPY-PUT analogs are specifically taken up via ATP13A3. We found that P5B-type ATPase isoforms transport fluorescently labeled polyamine analogs with a distinct structure-activity relationship (SAR), suggesting that isoform-specific polyamine probes can be designed.
Keywords: P5B-type ATPases; fluorescently labeled polyamines; mammalian polyamine transport systems; radiolabeled polyamines.
Conflict of interest statement
The authors declare no conflict of interest. KU Leuven has granted EMD Millipore Corporation an exclusive license for the commercialization of BODIPY-PUT, BODIPY-SPD and BODIPY-SPM described in this study.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

