Proton Migration on Top of Charged Membranes
- PMID: 36830721
- PMCID: PMC9953355
- DOI: 10.3390/biom13020352
Proton Migration on Top of Charged Membranes
Abstract
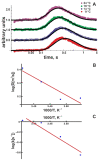
Proton relay between interfacial water molecules allows rapid two-dimensional diffusion. An energy barrier, ΔGr‡, opposes proton-surface-to-bulk release. The ΔGr‡-regulating mechanism thus far has remained unknown. Here, we explored the effect interfacial charges have on ΔGr‡'s enthalpic and entropic constituents, ΔGH‡ and ΔGS‡, respectively. A light flash illuminating a micrometer-sized membrane patch of a free-standing planar lipid bilayer released protons from an adsorbed hydrophobic caged compound. A lipid-anchored pH-sensitive dye reported protons' arrival at a distant membrane patch. Introducing net-negative charges to the bilayer doubled ΔGH‡, while positive net charges decreased ΔGH‡. The accompanying variations in ΔGS‡ compensated for the ΔGH‡ modifications so that ΔGr‡ was nearly constant. The increase in the entropic component of the barrier is most likely due to the lower number and strength of hydrogen bonds known to be formed by positively charged residues as compared to negatively charged moieties. The resulting high ΔGr‡ ensured interfacial proton diffusion for all measured membranes. The observation indicates that the variation in membrane surface charge alone is a poor regulator of proton traffic along the membrane surface.
Keywords: bioenergetics; caged proton; fluorimetry; interfacial proton diffusion; local proton circuit; proton transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

