Cytochrome P450 Surface Domains Prevent the β-Carotene Monohydroxylase CYP97H1 of Euglena gracilis from Acting as a Dihydroxylase
- PMID: 36830734
- PMCID: PMC9953315
- DOI: 10.3390/biom13020366
Cytochrome P450 Surface Domains Prevent the β-Carotene Monohydroxylase CYP97H1 of Euglena gracilis from Acting as a Dihydroxylase
Abstract
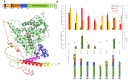
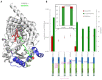
Molecular biodiversity results from branched metabolic pathways driven by enzymatic regioselectivities. An additional complexity occurs in metabolites with an internal structural symmetry, offering identical extremities to the enzymes. For example, in the terpene family, β-carotene presents two identical terminal closed-ring structures. Theses cycles can be hydroxylated by cytochrome P450s from the CYP97 family. Two sequential hydroxylations lead first to the formation of monohydroxylated β-cryptoxanthin and subsequently to that of dihydroxylated zeaxanthin. Among the CYP97 dihydroxylases, CYP97H1 from Euglena gracilis has been described as the only monohydroxylase. This study aims to determine which enzymatic domains are involved in this regioselectivity, conferring unique monohydroxylase activity on a substrate offering two identical sites for hydroxylation. We explored the effect of truncations, substitutions and domain swapping with other CYP97 members and found that CYP97H1 harbours a unique N-terminal globular domain. This CYP97H1 N-terminal domain harbours a hydrophobic patch at the entrance of the substrate channel, which is involved in the monohydroxylase activity of CYP97H1. This domain, at the surface of the enzyme, highlights the role of distal and non-catalytic domains in regulating enzyme specificity.
Keywords: asymmetric catalysis; carotenoids; cytochrome P450; protein engineering; regioselectivity; β-cryptoxanthin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
β-Cryptoxanthin Production in Escherichia coli by Optimization of the Cytochrome P450 CYP97H1 Activity.J Agric Food Chem. 2023 Mar 22;71(11):4683-4695. doi: 10.1021/acs.jafc.2c08970. Epub 2023 Mar 8. J Agric Food Chem. 2023. PMID: 36888893
-
Physiological role of β-carotene monohydroxylase (CYP97H1) in carotenoid biosynthesis in Euglena gracilis.Plant Sci. 2019 Jan;278:80-87. doi: 10.1016/j.plantsci.2018.10.017. Epub 2018 Nov 3. Plant Sci. 2019. PMID: 30471732
-
Zeaxanthin is required for eyespot formation and phototaxis in Euglena gracilis.Plant Physiol. 2023 Apr 3;191(4):2414-2426. doi: 10.1093/plphys/kiad001. Plant Physiol. 2023. PMID: 36611254 Free PMC article.
-
P450BM-3; a tale of two domains--or is it three?Steroids. 1997 Jan;62(1):117-23. doi: 10.1016/s0039-128x(96)00169-9. Steroids. 1997. PMID: 9029725 Review.
-
Differential behavior of the sub-sites of cytochrome 450 active site in binding of substrates, and products (implications for coupling/uncoupling).Biochim Biophys Acta. 2007 Mar;1770(3):360-75. doi: 10.1016/j.bbagen.2006.09.018. Epub 2006 Oct 5. Biochim Biophys Acta. 2007. PMID: 17134838 Review.
Cited by
-
Genome-wide characterization of the cytochrome P450 gene family in Solanum melongena and functional analysis of SmCYP82C1 under cold stress.BMC Plant Biol. 2025 Aug 2;25(1):1018. doi: 10.1186/s12870-025-07047-y. BMC Plant Biol. 2025. PMID: 40753368 Free PMC article.
References
-
- Biosynthesis of Carotenoids and Apocarotenoids by Microorganisms and Their Industrial Potential|IntechOpen. [(accessed on 7 November 2022)]. Available online: https://www.intechopen.com/chapters/62140.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

