Single-Cell RNA-Seq Analysis Reveals Macrophages Are Involved in the Pathogenesis of Human Sporadic Acute Type A Aortic Dissection
- PMID: 36830768
- PMCID: PMC9952989
- DOI: 10.3390/biom13020399
Single-Cell RNA-Seq Analysis Reveals Macrophages Are Involved in the Pathogenesis of Human Sporadic Acute Type A Aortic Dissection
Abstract
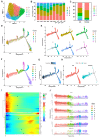
Macrophages play an important role in the progression of sporadic acute type A aortic dissection (ATAAD). The aim of this study was to characterize the cellular heterogeneity of macrophages in ATAAD tissues by scRNA-seq. Ascending aortic wall tissue from six ATAAD patients and three heart transplant donors was assessed by scRNA-seq and then analyzed and validated by various bioinformatic algorithms and histopathology experiments. The results revealed that the proportion of macrophages in ATAAD tissues (24.51%) was significantly higher than that in normal tissues (13.69%). Among the six macrophage subclusters, pro-inflammatory macrophages accounted for 14.96% of macrophages in the AD group and 0.18% in the normal group. Chemokine- and inflammation-related genes (CCL2, CCL20, S100A8, and S100A9) were expressed more intensively in macrophages in ATAAD tissue than in those in normal tissue. Additionally, intercellular communication analysis and transcription factor analysis indicated the activation of inflammation and degradation of the extracellular matrix in ATAAD tissue. Finally, immunohistochemistry, immunofluorescence, and Western blot experiments confirmed the overexpression of macrophage marker genes (CD68 and CD163) and matrix metalloproteinases (MMP9 and MMP2) in ATAAD tissue. Collectively, our study provides a preliminary evaluation of the role of macrophages in ATAAD, and the results could aid in the development of therapeutic options in the future.
Keywords: MMP2; MMP9; acute type A aortic dissection; inflammation; macrophage; matrix metalloproteinase; single-cell RNA sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Harris K.M., Nienaber C.A., Peterson M.D., Woznicki E.M., Braverman A.C., Trimarchi S., Myrmel T., Pyeritz R., Hutchison S., Strauss C., et al. Early Mortality in Type A Acute Aortic Dissection: Insights From the International Registry of Acute Aortic Dissection. JAMA Cardiol. 2022;7:1009–1015. doi: 10.1001/jamacardio.2022.2718. - DOI - PMC - PubMed
-
- Zhu Y., Lingala B., Baiocchi M., Tao J.J., Toro Arana V., Khoo J.W., Williams K.M., Traboulsi A.A., Hammond H.C., Lee A.M., et al. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J. Am. Coll. Cardiol. 2020;76:1703–1713. doi: 10.1016/j.jacc.2020.07.061. - DOI - PubMed
-
- Ye J., Wang M., Jiang H., Ji Q., Huang Y., Liu J., Zeng T., Xu Y., Wang Z., Lin Y., et al. Increased levels of interleukin-22 in thoracic aorta and plasma from patients with acute thoracic aortic dissection. Clin. Chim. Acta Int. J. Clin. Chem. 2018;486:395–401. doi: 10.1016/j.cca.2017.10.033. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- 81600245/the National Natural Science Foundation of China-Youth Science Fund
- 2022A1515011041/The General Program of Natural Science Foundation of Guangdong Province
- 1320900026/3×3 Clinical Scientist Fund of Sun Yat-sen Memorial Hospital
- YXQH202203/The General Program of Sun Yat Sen Sailing Foundation
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

