Hepatitis C Virus-Lipid Interplay: Pathogenesis and Clinical Impact
- PMID: 36830808
- PMCID: PMC9953247
- DOI: 10.3390/biomedicines11020271
Hepatitis C Virus-Lipid Interplay: Pathogenesis and Clinical Impact
Abstract
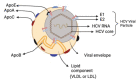
Hepatitis C virus (HCV) infection represents the major cause of chronic liver disease, leading to a wide range of hepatic diseases, including cirrhosis and hepatocellular carcinoma. It is the leading indication for liver transplantation worldwide. In addition, there is a growing body of evidence concerning the role of HCV in extrahepatic manifestations, including immune-related disorders and metabolic abnormalities, such as insulin resistance and steatosis. HCV depends on its host cells to propagate successfully, and every aspect of the HCV life cycle is closely related to human lipid metabolism. The virus circulates as a lipid-rich particle, entering the hepatocyte via lipoprotein cell receptors. It has also been shown to upregulate lipid biosynthesis and impair lipid degradation, resulting in significant intracellular lipid accumulation (steatosis) and circulating hypocholesterolemia. Patients with chronic HCV are at increased risk for hepatic steatosis, dyslipidemia, and cardiovascular disease, including accelerated atherosclerosis. This review aims to describe different aspects of the HCV viral life cycle as it impacts host lipoproteins and lipid metabolism. It then discusses the mechanisms of HCV-related hepatic steatosis, hypocholesterolemia, and accelerated atherosclerosis.
Keywords: atherosclerosis; cholesterol; hepatitis C; lipid metabolism; steatosis.
Conflict of interest statement
GS has acted as a speaker for Merck, Gilead, Abbvie, Novonordisk, Novartis and Pfizer, served as an advisory board member for Pfizer, Merck, Novonordisk, Gilead and Intercept, and has received unrestricted research funding from Theratec. NK reports research funding from Gilead Sciences, advisory fees from Gilead Sciences, ViiV Healthcare, Merck and Abbvie, and speaker fees from Gilead Sciences and Merck. TC and WE declare no conflict of interest.
Figures



References
-
- d’Avigdor W.M.H., Budzinska M.A., Lee M., Lam R., Kench J., Stapelberg M., McLennan S.V., Farrell G., George J., McCaughan G.W., et al. Virus Genotype-Dependent Transcriptional Alterations in Lipid Metabolism and Inflammation Pathways in the Hepatitis C Virus-infected Liver. Sci. Rep. 2019;9:10596. doi: 10.1038/s41598-019-46664-0. - DOI - PMC - PubMed
-
- Duan X., Anwar M.I., Xu Z., Ma L., Yuan G., Chen Y., Xi L., Jinyu X., Yuanping Z., Li Y.-P. Adaptive mutation F772S-enhanced p7-NS4A cooperation facilitates the assembly and release of hepatitis C virus and is associated with lipid droplet enlargement. Emerg. Microbes Infect. 2018;7:143. doi: 10.1038/s41426-018-0140-z. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

