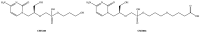Oral Brincidofovir Therapy for Monkeypox Outbreak: A Focused Review on the Therapeutic Potential, Clinical Studies, Patent Literature, and Prospects
- PMID: 36830816
- PMCID: PMC9953536
- DOI: 10.3390/biomedicines11020278
Oral Brincidofovir Therapy for Monkeypox Outbreak: A Focused Review on the Therapeutic Potential, Clinical Studies, Patent Literature, and Prospects
Abstract
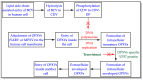
The monkeypox disease (MPX) outbreak of 2022 has been reported in more than one hundred countries and is becoming a global concern. Unfortunately, only a few treatments, such as tecovirimat (TCV), are available against MPX. Brincidofovir (BCV) is a United States Food and Drug Administration (USFDA)-approved antiviral against smallpox. This article reviews the potential of BCV for treating MPX and other Orthopoxvirus (OPXVs) diseases. The literature for this review was collected from PubMed, authentic websites (USFDA, Chimerix), and freely available patent databases (USPTO, Espacenet, and Patentscope). BCV (a lipophilic derivative of cidofovir) has been discovered and developed by Chimerix Incorporation, USA. Besides smallpox, BCV has also been tested clinically for various viral infections (adenovirus, cytomegalovirus, ebola virus, herpes simplex virus, and double-stranded DNA virus). Many health agencies and reports have recommended using BCV for MPX. However, no health agency has yet approved BCV for MPX. Accordingly, the off-label use of BCV is anticipated for MPX and various viral diseases. The patent literature revealed some important antiviral compositions of BCV. The authors believe there is a huge opportunity to create novel, inventive, and patentable BCV-based antiviral therapies (new combinations with existing antivirals) for OPXVs illnesses (MPX, smallpox, cowpox, camelpox, and vaccinia). It is also advised to conduct drug interaction (food, drug, and disease interaction) and drug resistance investigations on BCV while developing its combinations with other medications. The BCV-based drug repurposing options are also open for further exploration. BCV offers a promising opportunity for biosecurity against OPXV-based bioterrorism attacks and to control the MPX outbreak of 2022.
Keywords: CMX001; Orthopoxvirus; brincidofovir; cidofovir; monkeypox; patent; smallpox.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Novel derivatives of brincidofovir and (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine inhibit orthopoxviruses and human adenoviruses more potently than brincidofovir.Signal Transduct Target Ther. 2025 Apr 11;10(1):114. doi: 10.1038/s41392-025-02207-w. Signal Transduct Target Ther. 2025. PMID: 40210872 Free PMC article.
-
A Glance at the Development and Patent Literature of Tecovirimat: The First-in-Class Therapy for Emerging Monkeypox Outbreak.Viruses. 2022 Aug 25;14(9):1870. doi: 10.3390/v14091870. Viruses. 2022. PMID: 36146675 Free PMC article. Review.
-
Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model.mSphere. 2021 Feb 3;6(1):e00927-20. doi: 10.1128/mSphere.00927-20. mSphere. 2021. PMID: 33536322 Free PMC article.
-
Human monkeypox infection threat: A comprehensive overview.PLoS Negl Trop Dis. 2023 Apr 20;17(4):e0011246. doi: 10.1371/journal.pntd.0011246. eCollection 2023 Apr. PLoS Negl Trop Dis. 2023. PMID: 37079486 Free PMC article. Review.
-
Efficacy of delayed brincidofovir treatment against a lethal rabbitpox virus challenge in New Zealand White rabbits.Antiviral Res. 2017 Jul;143:278-286. doi: 10.1016/j.antiviral.2017.04.002. Epub 2017 Apr 7. Antiviral Res. 2017. PMID: 28392420
Cited by
-
Clinical Predictors of Monkeypox Diagnosis: A Case-Control Study in a Nonendemic Region during the 2022 Outbreak.Microorganisms. 2023 Sep 11;11(9):2287. doi: 10.3390/microorganisms11092287. Microorganisms. 2023. PMID: 37764131 Free PMC article.
-
Mpox (formerly monkeypox): pathogenesis, prevention, and treatment.Signal Transduct Target Ther. 2023 Dec 27;8(1):458. doi: 10.1038/s41392-023-01675-2. Signal Transduct Target Ther. 2023. PMID: 38148355 Free PMC article. Review.
-
Monkeypox: Origin, Transmission, Clinical Manifestations, Prevention, and Therapeutic Options.Interdiscip Perspect Infect Dis. 2025 Feb 2;2025:2522741. doi: 10.1155/ipid/2522741. eCollection 2025. Interdiscip Perspect Infect Dis. 2025. PMID: 39950190 Free PMC article. Review.
-
Drug screen reveals new potent host-targeted antivirals against Mpox virus.bioRxiv [Preprint]. 2025 May 5:2025.05.02.651913. doi: 10.1101/2025.05.02.651913. bioRxiv. 2025. PMID: 40400715 Free PMC article. Preprint.
-
Novel derivatives of brincidofovir and (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine inhibit orthopoxviruses and human adenoviruses more potently than brincidofovir.Signal Transduct Target Ther. 2025 Apr 11;10(1):114. doi: 10.1038/s41392-025-02207-w. Signal Transduct Target Ther. 2025. PMID: 40210872 Free PMC article.
References
-
- Almehmadi M., Allahyani M., Alsaiari A.A., Alshammari M.K., Alharbim A.S., Hussain K.H., Alsubaihi L.I., Kamal M., Alotaibi S.S., Alotaibi A.N., et al. A glance at the development and patent literature of tecovirimat: The first-in-class therapy for emerging monkeypox outbreak. Viruses. 2022;14:1870. doi: 10.3390/v14091870. - DOI - PMC - PubMed
-
- Multi-Country Outbreak of Monkeypox, External Situation Report #8. [(accessed on 29 October 2022)]. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-monkey....
-
- Moore M., Zahra F. StatPearls [Internet] StatPearls Publishing; Tampa, FL, USA: 2022. Monkeypox.
Publication types
LinkOut - more resources
Full Text Sources