Mutual Influence of Human Cytochrome P450 Enzymes and UDP-Glucuronosyltransferases on Their Respective Activities in Recombinant Fission Yeast
- PMID: 36830817
- PMCID: PMC9953201
- DOI: 10.3390/biomedicines11020281
Mutual Influence of Human Cytochrome P450 Enzymes and UDP-Glucuronosyltransferases on Their Respective Activities in Recombinant Fission Yeast
Abstract
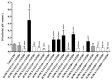
Cytochromes P450 (CYPs) and UDP-glucuronosyltransferases (UGTs) are the most important human drug metabolizing enzymes, but their mutual interactions are poorly understood. In this study, we recombinantly co-expressed of each one of the 19 human members of the UGT families 1 and 2 with either CYP2C9, CYP2D6, or CYP4Z1 in fission yeast. Using these strains, we monitored a total of 72 interactions: 57 cases where we tested the influence of UGT co-expression on CYP activity and 15 cases of the opposite approach. In the majority of cases (88%), UGT co-expression had a statistically significant (p < 0.05) effect on P450 activity (58% positive and 30% negative). Strong changes were observed in nine cases, including one case with an activity increase by a factor of 23 (CYP2C9 activity in the presence of UGT2A3) but also four cases with a complete loss of activity. When monitoring the effect of CYP co-expression on the activity of five UGTs, activity changes were generally not so pronounced and, if observed, always detrimental. UGT2B7 activity was not influenced by CYP co-expression, while the other UGTs were affected to varying degrees. These data suggest the notion that mutual influence of CYPs and UGTs on each other's activity is a widespread phenomenon.
Keywords: CYP2C9; CYP2D6; CYP4Z1; Cytochrome P450; UDP-glucuronosyltransferase; UGT2B7.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Mutual Modulation of the Activities of Human CYP2D6 and Four UGTs during the Metabolism of Propranolol.Curr Issues Mol Biol. 2023 Aug 26;45(9):7130-7146. doi: 10.3390/cimb45090451. Curr Issues Mol Biol. 2023. PMID: 37754235 Free PMC article.
-
Protein-protein interactions between rat hepatic cytochromes P450 (P450s) and UDP-glucuronosyltransferases (UGTs): evidence for the functionally active UGT in P450-UGT complex.Drug Metab Pharmacokinet. 2007 Oct;22(5):367-76. doi: 10.2133/dmpk.22.367. Drug Metab Pharmacokinet. 2007. PMID: 17965520
-
Direct comparison of UDP-glucuronosyltransferase and cytochrome P450 activities in human liver microsomes, plated and suspended primary human hepatocytes from five liver donors.Eur J Pharm Sci. 2017 Nov 15;109:96-110. doi: 10.1016/j.ejps.2017.07.032. Epub 2017 Aug 1. Eur J Pharm Sci. 2017. PMID: 28778465
-
Renal drug metabolism in humans: the potential for drug-endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT).Br J Clin Pharmacol. 2013 Oct;76(4):587-602. doi: 10.1111/bcp.12086. Br J Clin Pharmacol. 2013. PMID: 23362865 Free PMC article. Review.
-
Target proteins in human autoimmunity: cytochromes P450 and UDP- glucuronosyltransferases.Can J Gastroenterol. 2000 May;14(5):429-39. doi: 10.1155/2000/910107. Can J Gastroenterol. 2000. PMID: 10851284 Review.
Cited by
-
Mutual Modulation of the Activities of Human CYP2D6 and Four UGTs during the Metabolism of Propranolol.Curr Issues Mol Biol. 2023 Aug 26;45(9):7130-7146. doi: 10.3390/cimb45090451. Curr Issues Mol Biol. 2023. PMID: 37754235 Free PMC article.
References
-
- Williams J.A., Hyland R., Jones B.C., Smith D.A., Hurst S., Goosen T.C., Peterkin V., Koup J.R., Ball S.E. Drug-drug interactions for UDP-glucuronosyltransferase substrates: A pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab. Dispos. 2004;32:1201–1208. doi: 10.1124/dmd.104.000794. - DOI - PubMed
-
- Bernhardt R. Cytochrome P-450. In: Lennarz W.J., Lane M.D., editors. Encyclopedia of Biological Chemistry. Elsevier; New York, NY, USA: 2004. pp. 544–549.
LinkOut - more resources
Full Text Sources

