Two Ways of Targeting a CD19 Positive Relapse of Acute Lymphoblastic Leukaemia after Anti-CD19 CAR-T Cells
- PMID: 36830882
- PMCID: PMC9953531
- DOI: 10.3390/biomedicines11020345
Two Ways of Targeting a CD19 Positive Relapse of Acute Lymphoblastic Leukaemia after Anti-CD19 CAR-T Cells
Abstract
Background: Therapeutic options for CD19+ relapses after anti-CD19 CAR-T cells are still debated; second infusion of anti-CD19 CAR-T cells, therapeutic antibodies, or targeted therapies can be discussed. Here, we explore the immunophenotyping and lysis sensitivity of CD19+ ALL relapse after anti-CD19 CAR-T cells and propose different therapeutic options for such a high-risk disease.
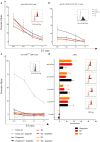
Methods: Cells from successive B-ALL relapses from one patient were collected. A broad immunophenotype analysis was performed. 51Cr cytotoxic assays, and long-term killing assays were conducted using T-cell effectors that are capable of cytotoxicity through three recognition pathways: antibody-dependent cell-mediated cytotoxicity (ADCC), anti-CD19 CAR-T, and TCR.
Results: Previously targeted antigen expression, even if maintained, decreased in relapses, and new targetable antigens appeared. Cytotoxic assays showed that ALL relapses remained sensitive to lysis mediated either by ADCC, CAR-T, or TCR, even if the lysis kinetics were different depending on the effector used. We also identified an immunosuppressive monocytic population in the last relapse sample that may have led to low persistence of CAR-T.
Conclusion: CD19+ relapses of ALL remain sensitive to cell lysis mediated by T-cell effectors. In case of ALL relapses after immunotherapy, a large immunophenotype will make new therapies possible for controlling such high risk ALL.
Keywords: CAR-T cells; acute lymphoblastic leukaemia; immunotherapy; relapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
-
- Dourthe M.E., Rabian F., Yakouben K., Chevillon F., Cabannes-Hamy A., Méchinaud F., Grain A., Chaillou D., Rahal I., Caillat-Zucman S., et al. Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia. 2021;35:3383–3393. doi: 10.1038/s41375-021-01281-7. - DOI - PubMed
-
- Grupp S.A., Maude S.L., Rives S., Baruchel A., Boyer M.W., Bittencourt H., Bader P., Büchner J., Laetsch T.W., Stefanski H., et al. Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (r/r) acute lymphoblastic leukemia. Blood. 2018;132:895. doi: 10.1182/blood-2018-99-112599. - DOI
LinkOut - more resources
Full Text Sources

