Magnetic Resonance Imaging of Primary Adult Brain Tumors: State of the Art and Future Perspectives
- PMID: 36830900
- PMCID: PMC9953338
- DOI: 10.3390/biomedicines11020364
Magnetic Resonance Imaging of Primary Adult Brain Tumors: State of the Art and Future Perspectives
Abstract
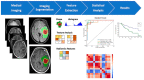
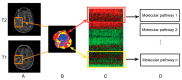
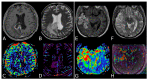
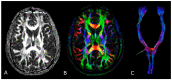
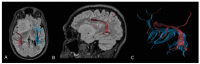
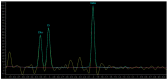
MRI is undoubtedly the cornerstone of brain tumor imaging, playing a key role in all phases of patient management, starting from diagnosis, through therapy planning, to treatment response and/or recurrence assessment. Currently, neuroimaging can describe morphologic and non-morphologic (functional, hemodynamic, metabolic, cellular, microstructural, and sometimes even genetic) characteristics of brain tumors, greatly contributing to diagnosis and follow-up. Knowing the technical aspects, strength and limits of each MR technique is crucial to correctly interpret MR brain studies and to address clinicians to the best treatment strategy. This article aimed to provide an overview of neuroimaging in the assessment of adult primary brain tumors. We started from the basilar role of conventional/morphological MR sequences, then analyzed, one by one, the non-morphological techniques, and finally highlighted future perspectives, such as radiomics and artificial intelligence.
Keywords: AI; DTI; MR spectroscopy; MRI; advanced MR Imaging; brain tumor imaging; functional MRI; perfusion MRI; quantitative MRI.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
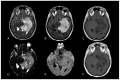
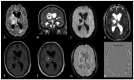
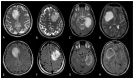
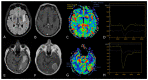

Similar articles
-
Advanced imaging techniques and non-invasive biomarkers in pediatric brain tumors: state of the art.Neuroradiology. 2024 Dec;66(12):2093-2116. doi: 10.1007/s00234-024-03476-y. Epub 2024 Oct 9. Neuroradiology. 2024. PMID: 39382639 Review.
-
Advancements in Neuroimaging to Unravel Biological and Molecular Features of Brain Tumors.Cancers (Basel). 2021 Jan 23;13(3):424. doi: 10.3390/cancers13030424. Cancers (Basel). 2021. PMID: 33498680 Free PMC article. Review.
-
Advanced Magnetic Resonance Imaging in the Evaluation of Treated Glioblastoma: A Pictorial Essay.Cancers (Basel). 2023 Jul 26;15(15):3790. doi: 10.3390/cancers15153790. Cancers (Basel). 2023. PMID: 37568606 Free PMC article. Review.
-
Advanced MR imaging in hemispheric low-grade gliomas before surgery; the indications and limits in the pediatric age.Childs Nerv Syst. 2016 Oct;32(10):1813-22. doi: 10.1007/s00381-016-3142-y. Epub 2016 Sep 20. Childs Nerv Syst. 2016. PMID: 27659824
-
Conventional and Advanced Imaging Techniques in Post-treatment Glioma Imaging.Front Radiol. 2022 Jun 28;2:883293. doi: 10.3389/fradi.2022.883293. eCollection 2022. Front Radiol. 2022. PMID: 37492665 Free PMC article. Review.
Cited by
-
Nanophotonic-enhanced photoacoustic imaging for brain tumor detection.J Nanobiotechnology. 2025 Mar 5;23(1):170. doi: 10.1186/s12951-025-03204-5. J Nanobiotechnology. 2025. PMID: 40045308 Free PMC article. Review.
-
Machine learning-based nomogram for distinguishing between supratentorial extraventricular ependymoma and supratentorial glioblastoma.Front Oncol. 2024 Sep 9;14:1443913. doi: 10.3389/fonc.2024.1443913. eCollection 2024. Front Oncol. 2024. PMID: 39319054 Free PMC article.
-
Preparation of surface-modified PLGA nanoparticles containing carbon quantum dots: insights from C6 cell line assays.Nanomedicine (Lond). 2025 Jun;20(12):1403-1416. doi: 10.1080/17435889.2025.2504322. Epub 2025 May 20. Nanomedicine (Lond). 2025. PMID: 40391996
-
Combination chemotherapy via poloxamer 188 surface-modified PLGA nanoparticles that traverse the blood-brain-barrier in a glioblastoma model.Sci Rep. 2024 Aug 22;14(1):19516. doi: 10.1038/s41598-024-69888-1. Sci Rep. 2024. PMID: 39174603 Free PMC article.
-
Is 18F-PSMA PET/CT a reliable imaging modality to evaluate the status of recurrent/residual brain tumors in post-treatment patients?J Neurooncol. 2025 Jul 31. doi: 10.1007/s11060-025-05167-x. Online ahead of print. J Neurooncol. 2025. PMID: 40745076
References
-
- Zhang B., MacFadden D., Damyanovich A.Z., Rieker M., Stainsby J., Bernstein M., Jaffray D.A., Mikulis D., Ménard C. Development of a geometrically accurate imaging protocol at 3 Tesla MRI for stereotactic radiosurgery treatment planning. Phys. Med. Biol. 2010;55:6601–6615. doi: 10.1088/0031-9155/55/22/002. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

