Peptides That Block RAS-p21 Protein-Induced Cell Transformation
- PMID: 36831007
- PMCID: PMC9953342
- DOI: 10.3390/biomedicines11020471
Peptides That Block RAS-p21 Protein-Induced Cell Transformation
Abstract
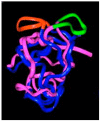
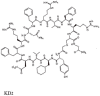
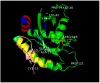
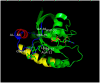
This is a review of approaches to the design of peptides and small molecules that selectively block the oncogenic RAS-p21 protein in ras-induced cancers. Single amino acid substitutions in this protein, at critical positions such as at Gly 12 and Gln 61, cause the protein to become oncogenic. These mutant proteins cause over 90 percent of pancreatic cancers, 40-50 percent of colon cancers and about one third of non-small cell cancers of the lung (NSCCL). RAS-p21 is a G-protein that becomes activated when it exchanges GDP for GTP. Several promising approaches have been developed that target mutant (oncogenic) RAS-p21 proteins in these different cancers. These approaches comprise: molecular simulations of mutant and wild-type proteins to identify effector domains, for which peptides can be made that selectively inhibit the oncogenic protein that include PNC-1 (ras residues 115-126), PNC-2 (ras residues 96-110) and PNC7 (ras residues 35-47); the use of contiguous RAS-p21 peptide sequences that can block ras signaling; cyclic peptides from large peptide libraries and small molecule libraries that can be identified in high throughput assays that can selectively stabilize inactive forms of RAS-p21; informatic approaches to discover peptides and small molecules that dock to specific domains of RAS-p21 that can block mitogenic signal transduction by oncogenic RAS-p21; and the use of cell-penetrating peptides (CPPs) that are attached to the variable domains of the anti-RAS-p21 inactivating monoclonal antibody, Y13 259, that selectively enters oncogenic RAS-p21-containing cancer cells, causing these cells to undergo apoptosis. Several new anti-oncogenic RAS-p21 agents, i.e., Amgen's AMG510 and Mirati Therapeutics' MRTX849, polycyclic aromatic compounds, have recently been FDA-approved and are already being used clinically to treat RAS-p21-induced NSCCL and colorectal carcinomas. These new drugs target the inactive form of RAS-p21 bound to GDP with G12C substitution at the critical Gly 12 residue by binding to a groove bordered by specific domains in this mutant protein into which these compounds insert, resulting in the stabilization of the inactive GDP-bound form of RAS-p21. Other peptides and small molecules have been discovered that block the G12D-RAS-p21 oncogenic protein. These agents can treat specific mutant protein-induced cancers and are excellent examples of personalized medicine. However, many oncogenic RAS-p21-induced tumors are caused by other mutations at positions 12, 13 and 61, requiring other, more general anti-oncogenic agents that are being provided using alternate methods.
Keywords: RAS-p21 protein; amino acid substitutions; blockade of oncogenic protein; cell transformation; mutant protein; oncogenic forms; peptides; small molecules.
Conflict of interest statement
None of the authors of this paper have any real or perceived conflicts of interest concerning any aspects of the work presented in this paper.
Figures



References
-
- Pincus M.R., Bluth M., Brandt-Rauf P.W., Bowne W., LaDoulis C. Oncoproteins and Early Tumor Detection, Chapter 77. In: McPherson R.A., Pincus M.R., editors. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 24th ed. Elsevier; New York, NY, USA: Philadelphia, PA, USA: 2021. pp. 1525–1541.
Publication types
LinkOut - more resources
Full Text Sources

