Oral Administration of Vitamin D3 Prevents Corneal Damage in a Knock-Out Mouse Model of Sjögren's Syndrome
- PMID: 36831152
- PMCID: PMC9953695
- DOI: 10.3390/biomedicines11020616
Oral Administration of Vitamin D3 Prevents Corneal Damage in a Knock-Out Mouse Model of Sjögren's Syndrome
Abstract
Background: Vitamin D deficiency has been associated with dry eye development during Sjögren's syndrome (SS). Here, we investigated whether repeated oral vitamin D3 supplementation could prevent the corneal epithelium damage in an SS mouse model.
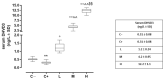
Methods: 30 female mouse knock-out for the thrombospondin 1 gene were randomized (six per group) in untreated mice euthanized at 6 weeks as negative control (C-) or at 12 weeks as the positive control for dry eye (C+). Other mice were sacrificed after 6 weeks of oral vitamin D3 supplementation in the drinking water (1000, 8000, and 20,000 IU/kg/week, respectively).
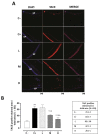
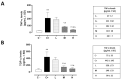
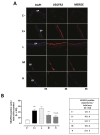
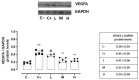
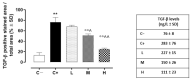
Results: The C+ mice showed alterations in their corneal epithelial morphologies and thicknesses (p < 0.01 vs. C-), while the mice receiving 8000 (M) and 20,000 (H) IU/kg/week of vitamin D3 showed preservation of the corneal epithelium morphology and thickness (p < 0.01 vs. C+). Moreover, while the C+ mice exhibited high levels and activity of corneal tumor necrosis factor alpha converting enzyme (TACE), neovascularization and fibrosis markers; these were all reduced in the M and H mice.
Conclusions: Oral vitamin D3 supplementation appeared to counteract the negative effect of TACE on corneal epithelium in a mouse model of SS-associated dry eye.
Keywords: Sjögren’s syndrome; cornea; dry eye; thrombospondin-1 knock-out mice; tumor necrosis factor alpha converting enzyme; vitamin D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rowe D., Griffiths M., Stewart J., Novick D., Beverley P.C., Isenberg D.A. HLA Class I and II, Interferon, Interleukin 2, and the Interleukin 2 Receptor Expression on Labial Biopsy Specimens from Patients with Sjogren’s Syndrome. Ann. Rheum. Dis. 1987;46:580–586. doi: 10.1136/ard.46.8.580. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

