The Role of Autophagy in Breast Cancer Metastasis
- PMID: 36831154
- PMCID: PMC9953203
- DOI: 10.3390/biomedicines11020618
The Role of Autophagy in Breast Cancer Metastasis
Abstract
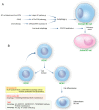
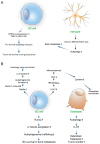
Patient morbidity and mortality is significantly increased in metastatic breast cancer. The metastasis process of breast cancer is very complicated and is delicately controlled by various factors. Autophagy is one of the important regulatory factors affecting metastasis in breast cancer by engaging in cell mobility, metabolic adaptation, tumor dormancy, and cancer stem cells. Here, we discuss the effects of autophagy on metastasis in breast cancer and assess the potential use of autophagy modulators for metastasis treatment.
Keywords: autophagy; autophagy modulators; breast cancer; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

