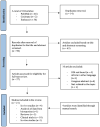
The Role of Growth Hormone and Insulin Growth Factor 1 in the Development of Non-Alcoholic Steato-Hepatitis: A Systematic Review
- PMID: 36831184
- PMCID: PMC9954460
- DOI: 10.3390/cells12040517
The Role of Growth Hormone and Insulin Growth Factor 1 in the Development of Non-Alcoholic Steato-Hepatitis: A Systematic Review
Abstract
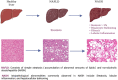
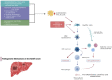
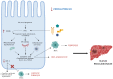
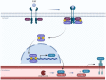
Diabetic and obese patients have a high prevalence of non-alcoholic fatty liver disease (NAFLD). This condition groups a spectrum of conditions varying from simple steatosis to non-alcoholic steatohepatitis (NASH), with or without fibrosis. Multiple factors are involved in the development of NAFLD. However, details about its pathogenesis and factors that promote the progression to NASH are still missing. Growth hormone (GH) and insulin-like growth factor 1 (IGF-1) regulate metabolic, immune, and hepatic stellate cell functions. Increasing evidence suggests they may have roles in the progression from NAFLD to NASH. Following the PRISMA reporting guidelines, we conducted a systematic review to evaluate all clinical and experimental studies published in the literature correlating GH and IGF-1 to inflammation and fibrosis in NAFLD and NASH. Our results showed that GH and IGF-1 have a fundamental role in the pathogenesis of NASH, acting in slightly different ways to produce a synergic effect. Indeed, GH may mediate its protective effect in the pathogenesis of NASH by regulating lipogenesis pathways, while IGF-1 has the same effect by regulating cholesterol transport. Therefore, they could be used as therapeutic strategies in preventing NAFLD progression to NASH.
Keywords: growth hormone; growth hormone receptor; insulin-like growth factor-1; non-alcoholic fatty liver disease; non-alcoholic steatohepatitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nishizawa H., Takahashi M., Fukuoka H., Iguchi G., Kitazawa R., Takahashi Y. GH-independent IGF-I action is essential to prevent the development of nonalcoholic steatohepatitis in a GH-deficient rat model. Biochem. Biophys. Res. Commun. 2012;423:295–300. doi: 10.1016/j.bbrc.2012.05.115. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

