Interplays of AMPK and TOR in Autophagy Regulation in Yeast
- PMID: 36831186
- PMCID: PMC9953913
- DOI: 10.3390/cells12040519
Interplays of AMPK and TOR in Autophagy Regulation in Yeast
Abstract
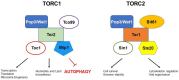
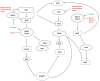
Cells survey their environment and need to balance growth and anabolism with stress programmes and catabolism towards maximum cellular bioenergetics economy and survival. Nutrient-responsive pathways, such as the mechanistic target of rapamycin (mTOR) interact and cross-talk, continuously, with stress-responsive hubs such as the AMP-activated protein kinase (AMPK) to regulate fundamental cellular processes such as transcription, protein translation, lipid and carbohydrate homeostasis. Especially in nutrient stresses or deprivations, cells tune their metabolism accordingly and, crucially, recycle materials through autophagy mechanisms. It has now become apparent that autophagy is pivotal in lifespan, health and cell survival as it is a gatekeeper of clearing damaged macromolecules and organelles and serving as quality assurance mechanism within cells. Autophagy is hard-wired with energy and nutrient levels as well as with damage-response, and yeasts have been instrumental in elucidating such connectivities. In this review, we briefly outline cross-talks and feedback loops that link growth and stress, mainly, in the fission yeast Schizosaccharomyces pombe, a favourite model in cell and molecular biology.
Keywords: S. pombe; ageing; caloric restriction; fission yeast; lifespan; mTOR; rapamycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

