Extracellular Vesicles in Aging: An Emerging Hallmark?
- PMID: 36831194
- PMCID: PMC9954704
- DOI: 10.3390/cells12040527
Extracellular Vesicles in Aging: An Emerging Hallmark?
Abstract
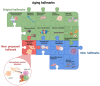
Extracellular vesicles (EVs) are membrane-enclosed particles secreted by cells and circulating in body fluids. Initially considered as a tool to dispose of unnecessary material, they are now considered an additional method to transmit cell signals. Aging is characterized by a progressive impairment of the physiological functions of tissues and organs. The causes of aging are complex and interconnected, but there is consensus that genomic instability, telomere erosion, epigenetic alteration, and defective proteostasis are primary hallmarks of the aging process. Recent studies have provided evidence that many of these primary stresses are associated with an increased release of EVs in cell models, able to spread senescence signals in the recipient cell. Additional investigations on the role of EVs during aging also demonstrated the great potential of EVs for the modulation of age-related phenotypes and for pro-rejuvenation therapies, potentially beneficial for many diseases associated with aging. Here we reviewed the current literature on EV secretion in senescent cell models and in old vs. young individual body fluids, as well as recent studies addressing the potential of EVs from different sources as an anti-aging tool. Although this is a recent field, the robust consensus on the altered EV release in aging suggests that altered EV secretion could be considered an emerging hallmark of aging.
Keywords: aging; extracellular vesicles (EVs); senescence; senescence-associated secretory phenotype (SASP).
Conflict of interest statement
The authors declare no conflict of interest
Figures

References
-
- Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Ves. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

