Up-Regulation of Astrocytic Fgfr4 Expression in Adult Mice after Spinal Cord Injury
- PMID: 36831195
- PMCID: PMC9954417
- DOI: 10.3390/cells12040528
Up-Regulation of Astrocytic Fgfr4 Expression in Adult Mice after Spinal Cord Injury
Abstract
Spinal cord injury (SCI) leads to persistent neurological deficits without available curative treatment. After SCI astrocytes within the lesion vicinity become reactive, these undergo major morphological, and molecular transformations. Previously, we reported that following SCI, over 10% of resident astrocytes surrounding the lesion spontaneously transdifferentiate towards a neuronal phenotype. Moreover, this conversion is associated with an increased expression of fibroblast growth factor receptor 4 (Fgfr4), a neural stem cell marker, in astrocytes. Here, we evaluate the therapeutic potential of gene therapy upon Fgfr4 over-expression in mature astrocytes following SCI in adult mice. We found that Fgfr4 over-expression in astrocytes immediately after SCI improves motor function recovery; however, it may display sexual dimorphism. Improved functional recovery is associated with a decrease in spinal cord lesion volume and reduced glial reactivity. Cell-specific transcriptomic profiling revealed concomitant downregulation of Notch signaling, and up-regulation of neurogenic pathways in converting astrocytes. Our findings suggest that gene therapy targeting Fgfr4 over-expression in astrocytes after injury is a feasible therapeutic approach to improve recovery following traumatism of the spinal cord. Moreover, we stress that a sex-dependent response to astrocytic modulation should be considered for the development of effective translational strategies in other neurological disorders.
Keywords: Fgfr4; astrocytes; gene therapy; spinal cord injury; transdifferentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
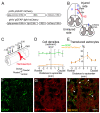
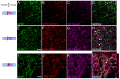
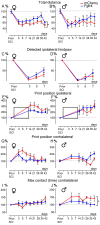
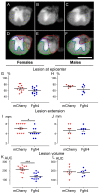

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

