Infrared Laser Effects on Cell Projection Depend on Irradiation Intermittence and Cell Activity
- PMID: 36831208
- PMCID: PMC9954793
- DOI: 10.3390/cells12040540
Infrared Laser Effects on Cell Projection Depend on Irradiation Intermittence and Cell Activity
Abstract
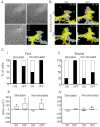
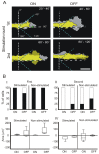
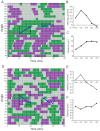
Highly focused near-infrared (NIR) lasers have been used to induce fibroblast and neuron protrusions in a technique called optical guidance. However, little is known about the biochemical and biophysical effects that the laser provokes in the cell and optimal protocols of stimulation have not yet been established. Using intermittent NIR laser radiation and multivariate time series representations of cell leading edge movement, we analyzed the direction and velocity of cell protrusions. We found that the orientation and advance of PC12 neuron phenotype cells and 3T3 fibroblasts protrusions remain after the laser is turned off, but the observed increase in velocity stops when radiation ceases. For an increase in the speed and distance of cell protrusions by NIR laser irradiation, the cell leading edge needs to be advancing prior to the stimulation, and NIR irradiation does not enable the cell to switch between retracting and advancing states. Using timelapse imaging of actin-GFP, we observed that NIR irradiation induces a faster recruitment of actin, promoting filament formation at the induced cell protrusions. These results provide fresh evidence to understand the phenomenon of the optical guidance of cell protrusions.
Keywords: actin; cells projection; optical guidance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Avila R., Medina-Villalobos N., Tamariz E., Chiu R., Lopez-Marín L.M., Acosta A., Castaño V. Optical tweezers experiments for fibroblast cell growth stimulation; Proceedings of the Biophotonics: Photonic Solutions for Better Health Care IV; Brussels, Belgium. 14–17 April 2014; p. 91291U. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

