The Role and Mechanism of Transglutaminase 2 in Regulating Hippocampal Neurogenesis after Traumatic Brain Injury
- PMID: 36831225
- PMCID: PMC9954100
- DOI: 10.3390/cells12040558
The Role and Mechanism of Transglutaminase 2 in Regulating Hippocampal Neurogenesis after Traumatic Brain Injury
Abstract
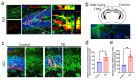
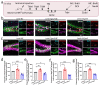
Traumatic brain injury usually results in neuronal loss and cognitive deficits. Promoting endogenous neurogenesis has been considered as a viable treatment option to improve functional recovery after TBI. However, neural stem/progenitor cells (NSPCs) in neurogenic regions are often unable to migrate and differentiate into mature neurons at the injury site. Transglutaminase 2 (TGM2) has been identified as a crucial component of neurogenic niche, and significantly dysregulated after TBI. Therefore, we speculate that TGM2 may play an important role in neurogenesis after TBI, and strategies targeting TGM2 to promote endogenous neural regeneration may be applied in TBI therapy. Using a tamoxifen-induced Tgm2 conditional knockout mouse line and a mouse model of stab wound injury, we investigated the role and mechanism of TGM2 in regulating hippocampal neurogenesis after TBI. We found that Tgm2 was highly expressed in adult NSPCs and up-regulated after TBI. Conditional deletion of Tgm2 resulted in the impaired proliferation and differentiation of NSPCs, while Tgm2 overexpression enhanced the abilities of self-renewal, proliferation, differentiation, and migration of NSPCs after TBI. Importantly, injection of lentivirus overexpressing TGM2 significantly promoted hippocampal neurogenesis after TBI. Therefore, TGM2 is a key regulator of hippocampal neurogenesis and a pivotal therapeutic target for intervention following TBI.
Keywords: hippocampus; neurogenesis; neuronal stem/progenitor cell; transglutaminase 2; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., Bragge P., Brazinova A., Buki A., Chesnut R.M., et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

