Direct Cell Reprogramming and Phenotypic Conversion: An Analysis of Experimental Attempts to Transform Astrocytes into Neurons in Adult Animals
- PMID: 36831283
- PMCID: PMC9954435
- DOI: 10.3390/cells12040618
Direct Cell Reprogramming and Phenotypic Conversion: An Analysis of Experimental Attempts to Transform Astrocytes into Neurons in Adult Animals
Abstract
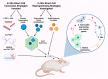
Central nervous system (CNS) repair after injury or disease remains an unresolved problem in neurobiology research and an unmet medical need. Directly reprogramming or converting astrocytes to neurons (AtN) in adult animals has been investigated as a potential strategy to facilitate brain and spinal cord recovery and advance fundamental biology. Conceptually, AtN strategies rely on forced expression or repression of lineage-specific transcription factors to make endogenous astrocytes become "induced neurons" (iNs), presumably without re-entering any pluripotent or multipotent states. The AtN-derived cells have been reported to manifest certain neuronal functions in vivo. However, this approach has raised many new questions and alternative explanations regarding the biological features of the end products (e.g., iNs versus neuron-like cells, neural functional changes, etc.), developmental biology underpinnings, and neurobiological essentials. For this paper per se, we proposed to draw an unconventional distinction between direct cell conversion and direct cell reprogramming, relative to somatic nuclear transfer, based on the experimental methods utilized to initiate the transformation process, aiming to promote a more in-depth mechanistic exploration. Moreover, we have summarized the current tactics employed for AtN induction, comparisons between the bench endeavors concerning outcome tangibility, and discussion of the issues of published AtN protocols. Lastly, the urgency to clearly define/devise the theoretical frameworks, cell biological bases, and bench specifics to experimentally validate primary data of AtN studies was highlighted.
Keywords: astrocyte; direct cell conversion; direct cell reprogramming; functional multipotency of stem cells; neurodegeneration; neuron; neurotrauma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hamilton A. The division of differentiated cells in the central nervous system of the white rat. J. Comp. Neurol. 1901;11:297–320. doi: 10.1002/cne.910110403. - DOI
-
- Kjell J., Fischer-Sternjak J., Thompson A.J., Friess C., Sticco M.J., Salinas F., Cox J., Martinelli D.C., Ninkovic J., Franze K., et al. Defining the Adult Neural Stem Cell Niche Proteome Identifies Key Regulators of Adult Neurogenesis. Cell Stem Cell. 2020;26:277–293. doi: 10.1016/j.stem.2020.01.002. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

