SERPIN-Derived Small Peptide (SP16) as a Potential Therapeutic Agent against HIV-Induced Inflammatory Molecules and Viral Replication in Cells of the Central Nervous System
- PMID: 36831299
- PMCID: PMC9954444
- DOI: 10.3390/cells12040632
SERPIN-Derived Small Peptide (SP16) as a Potential Therapeutic Agent against HIV-Induced Inflammatory Molecules and Viral Replication in Cells of the Central Nervous System
Abstract
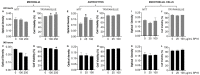
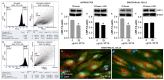
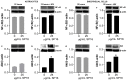
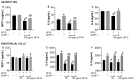
Despite the success of combined antiretroviral therapy (cART) increasing the survival rate in human immunodeficiency virus (HIV) patients, low levels of viremia persist in the brain of patients leading to glia (microglia and astrocytes)-induced neuroinflammation and consequently, the reactivation of HIV and neuronal injury. Here, we tested the therapeutic efficacy of a Low-Density Lipoprotein Receptor-Related Protein 1 (LRP-1) agonistic small peptide drug (SP16) in attenuating HIV replication and the secretion of inflammatory molecules in brain reservoirs. SP16 was developed by Serpin Pharma and is derived from the pentapeptide sequence of the serine protease inhibitor alpha-1-antitrypsin (A1AT). The SP16 peptide sequence was subsequently modified to improve the stability, bioavailability, efficacy, and binding to LRP-1; a scavenger regulatory receptor that internalizes ligands to induce anti-viral, anti-inflammatory, and pro-survival signals. Using glial cells infected with HIV, we showed that: (i) SP16 attenuated viral-induced secretion of pro-inflammatory molecules; and (ii) SP16 attenuated viral replication. Using an artificial 3D blood-brain barrier (BBB) system, we showed that: (i) SP16 was transported across the BBB; and (ii) restored the permeability of the BBB compromised by HIV. Mechanistically, we showed that SP16 interaction with LRP-1 and binding lead to: (i) down-regulation in the expression levels of nuclear factor-kappa beta (NF-κB); and (ii) up-regulation in the expression levels of Akt. Using an in vivo mouse model, we showed that SP16 was transported across the BBB after intranasal delivery, while animals infected with EcoHIV undergo a reduction in (i) viral replication and (ii) viral secreted inflammatory molecules, after exposure to SP16 and antiretrovirals. Overall, these studies confirm a therapeutic response of SP16 against HIV-associated inflammatory effects in the brain.
Keywords: blood-brain barrier; intranasal delivery; low-density lipoprotein receptor related-protein 1; serine protease inhibitors; therapeutic efficacy.
Conflict of interest statement
Cohava Gelber is the CEO of Serpin Pharma. The authors declare that the research was conducted in the absence of any commercial or financial relationships from Serpin Pharma, that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

