Administration of Secretome Derived from Human Mesenchymal Stem Cells Induces Hepatoprotective Effects in Models of Idiosyncratic Drug-Induced Liver Injury Caused by Amiodarone or Tamoxifen
- PMID: 36831304
- PMCID: PMC9954258
- DOI: 10.3390/cells12040636
Administration of Secretome Derived from Human Mesenchymal Stem Cells Induces Hepatoprotective Effects in Models of Idiosyncratic Drug-Induced Liver Injury Caused by Amiodarone or Tamoxifen
Abstract
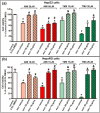
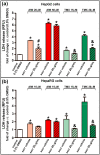
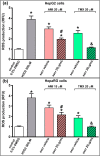
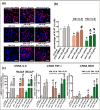
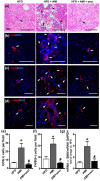
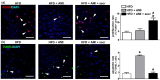
Drug-induced liver injury (DILI) is one of the leading causes of acute liver injury. While many factors may contribute to the susceptibility to DILI, obese patients with hepatic steatosis are particularly prone to suffer DILI. The secretome derived from mesenchymal stem cell has been shown to have hepatoprotective effects in diverse in vitro and in vivo models. In this study, we evaluate whether MSC secretome could improve DILI mediated by amiodarone (AMI) or tamoxifen (TMX). Hepatic HepG2 and HepaRG cells were incubated with AMI or TMX, alone or with the secretome of MSCs obtained from human adipose tissue. These studies demonstrate that coincubation of AMI or TMX with MSC secretome increases cell viability, prevents the activation of apoptosis pathways, and stimulates the expression of priming phase genes, leading to higher proliferation rates. As proof of concept, in a C57BL/6 mouse model of hepatic steatosis and chronic exposure to AMI, the MSC secretome was administered endovenously. In this study, liver injury was significantly attenuated, with a decrease in cell infiltration and stimulation of the regenerative response. The present results indicate that MSC secretome administration has the potential to be an adjunctive cell-free therapy to prevent liver failure derived from DILI caused by TMX or AMI.
Keywords: amiodarone; cell free therapy; drug-induced liver injury; hepatic regeneration; tamoxifen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury.Stem Cell Res Ther. 2018 Feb 6;9(1):31. doi: 10.1186/s13287-017-0752-6. Stem Cell Res Ther. 2018. PMID: 29409540 Free PMC article.
-
Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models.Stem Cell Res Ther. 2014 Jun 10;5(3):76. doi: 10.1186/scrt465. Stem Cell Res Ther. 2014. PMID: 24915963 Free PMC article.
-
Osthole prevents tamoxifen-induced liver injury in mice.Acta Pharmacol Sin. 2019 May;40(5):608-619. doi: 10.1038/s41401-018-0171-y. Epub 2018 Oct 12. Acta Pharmacol Sin. 2019. PMID: 30315252 Free PMC article.
-
New Perspectives to Improve Mesenchymal Stem Cell Therapies for Drug-Induced Liver Injury.Int J Mol Sci. 2022 Feb 28;23(5):2669. doi: 10.3390/ijms23052669. Int J Mol Sci. 2022. PMID: 35269830 Free PMC article. Review.
-
Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome.Cells. 2019 May 16;8(5):467. doi: 10.3390/cells8050467. Cells. 2019. PMID: 31100966 Free PMC article. Review.
Cited by
-
Mesenchymal stromal cells alleviate APAP-induced liver injury via extracellular vesicle-mediated regulation of the miR-186-5p/CXCL1 axis.Stem Cell Res Ther. 2024 Nov 3;15(1):392. doi: 10.1186/s13287-024-03995-8. Stem Cell Res Ther. 2024. PMID: 39490995 Free PMC article.
-
Biomaterial Fg/P(LLA-CL) regulates macrophage polarization and recruitment of mesenchymal stem cells after endometrial injury.J Mater Sci Mater Med. 2024 Jul 29;35(1):39. doi: 10.1007/s10856-024-06807-w. J Mater Sci Mater Med. 2024. PMID: 39073624 Free PMC article.
-
The Immunoregulatory and Regenerative Potential of Activated Human Stem Cell Secretome Mitigates Acute-on-Chronic Liver Failure in a Rat Model.Int J Mol Sci. 2024 Feb 8;25(4):2073. doi: 10.3390/ijms25042073. Int J Mol Sci. 2024. PMID: 38396750 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

