Implications of Senescent Cell Burden and NRF2 Pathway in Uremic Calcification: A Translational Study
- PMID: 36831311
- PMCID: PMC9954542
- DOI: 10.3390/cells12040643
Implications of Senescent Cell Burden and NRF2 Pathway in Uremic Calcification: A Translational Study
Abstract
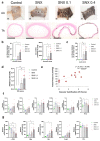
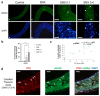
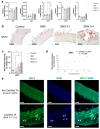
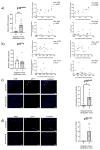
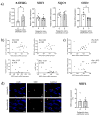
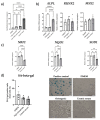
Increased senescent cell burden and dysregulation of the nuclear factor erythroid 2-related factor 2 (NRF2) pathway have been associated with numerous age-related pathologies; however, their role in promoting vascular calcification (VC) in chronic kidney disease (CKD) has yet to be determined. We investigated whether senescence and NRF2 pathways may serve as drivers of uremia-induced VC using three complementary approaches: a novel model of induced VC in 5/6-nephrectomized rats supplemented with high phosphate and vitamin D; epigastric arteries from CKD patients with established medial calcification; and vascular smooth muscle cells (VSMCs) incubated with uremic serum. Expression of p16Ink4a and p21Cip1, as well as γ-H2A-positive cells, confirmed increased senescent cell burden at the site of calcium deposits in aortic sections in rats, and was similarly observed in calcified epigastric arteries from CKD patients through increased p16Ink4a expression. However, uremic serum-induced VSMC calcification was not accompanied by senescence. Expression of NRF2 and downstream genes, Nqo1 and Sod1, was associated with calcification in uremic rats, while no difference was observed between calcified and non-calcified EAs. Conversely, in vitro uremic serum-driven VC was associated with depleted NRF2 expression. Together, our data strengthen the importance of senescence and NRF2 pathways as potential therapeutic options to combat VC in CKD.
Keywords: NRF2; kidney failure; senescence; subtotal nephrectomy; vascular calcification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- 3. Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., Afarideh M., Agarwal S.K., Agudelo-Botero M., et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. - DOI - PMC - PubMed
-
- Chen J., Budoff M.J., Reilly M., Yang W., Rosas S.E., Rahman M., Zhang X., Roy J.A., Lustigova E., Nessel L., et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients With Chronic Kidney Disease. JAMA Cardiol. 2017;2:635–643. doi: 10.1001/jamacardio.2017.0363. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

