Extracellular Vesicles and MicroRNA in Myelodysplastic Syndromes
- PMID: 36831325
- PMCID: PMC9955013
- DOI: 10.3390/cells12040658
Extracellular Vesicles and MicroRNA in Myelodysplastic Syndromes
Abstract
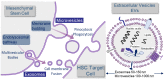
The bone marrow niche plays an increasing role in the pathophysiogenesis of myelodysplastic syndromes. More specifically, mesenchymal stromal cells, which can secrete extracellular vesicles and their miRNA contents, modulate the fate of hematopoietic stem cells leading to leukemogenesis. Extracellular vesicles can mediate their miRNA and protein contents between nearby cells but also in the plasma of the patients, being potent tools for diagnosis and prognostic markers in MDS. They can be targeted by antisense miRNA or by modulators of the secretion of extracellular vesicles and could lead to future therapeutic directions in MDS.
Keywords: bone marrow niche; exosomes; extracellular vesicles; miRNA; microvesicles; myelodysplastic syndromes; non-coding RNA.
Conflict of interest statement
David Laurin was employed by the French blood bank “Etablissement Français du Sang” (EFS), a non-profit public institution. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bersanelli M., Travaglino E., Meggendorfer M., Matteuzzi T., Sala C., Mosca E., Chiereghin C., Di Nanni N., Gnocchi M., Zampini M., et al. Classification and Personalized Prognostic Assessment on the Basis of Clinical and Genomic Features in Myelodysplastic Syndromes. J. Clin. Oncol. 2021;39:1223–1233. doi: 10.1200/JCO.20.01659. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

