A Wrong Fate Decision in Adipose Stem Cells upon Obesity
- PMID: 36831329
- PMCID: PMC9954614
- DOI: 10.3390/cells12040662
A Wrong Fate Decision in Adipose Stem Cells upon Obesity
Abstract
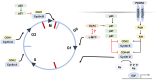
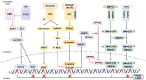
Progress has been made in identifying stem cell aging as a pathological manifestation of a variety of diseases, including obesity. Adipose stem cells (ASCs) play a core role in adipocyte turnover, which maintains tissue homeostasis. Given aberrant lineage determination as a feature of stem cell aging, failure in adipogenesis is a culprit of adipose hypertrophy, resulting in adiposopathy and related complications. In this review, we elucidate how ASC fails in entering adipogenic lineage, with a specific focus on extracellular signaling pathways, epigenetic drift, metabolic reprogramming, and mechanical stretch. Nonetheless, such detrimental alternations can be reversed by guiding ASCs towards adipogenesis. Considering the pathological role of ASC aging in obesity, targeting adipogenesis as an anti-obesity treatment will be a key area of future research, and a strategy to rejuvenate tissue stem cell will be capable of alleviating metabolic syndrome.
Keywords: adipogenesis; fate determination; hypertrophic obesity; inflammation; stem cell aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vishvanath L., MacPherson K.A., Hepler C., Wang Q.A., Shao M., Spurgin S.B., Wang M.Y., Kusminski C.M., Morley T.S., Gupta R.K. Pdgfrβ+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell. Metab. 2016;23:350–359. doi: 10.1016/j.cmet.2015.10.018. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

