Sirtuin 6-A Key Regulator of Hepatic Lipid Metabolism and Liver Health
- PMID: 36831330
- PMCID: PMC9954390
- DOI: 10.3390/cells12040663
Sirtuin 6-A Key Regulator of Hepatic Lipid Metabolism and Liver Health
Abstract
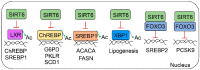
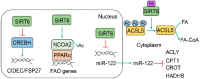
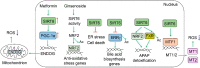
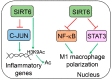
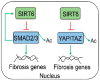
Sirtuin 6 (SIRT6) is an NAD-dependent deacetylase/deacylase/mono-ADP ribosyltransferase, a member of the sirtuin protein family. SIRT6 has been implicated in hepatic lipid homeostasis and liver health. Hepatic lipogenesis is driven by several master regulators including liver X receptor (LXR), carbohydrate response element binding protein (ChREBP), and sterol regulatory element binding protein 1 (SREBP1). Interestingly, these three transcription factors can be negatively regulated by SIRT6 through direct deacetylation. Fatty acid oxidation is regulated by peroxisome proliferator activated receptor alpha (PPARα) in the liver. SIRT6 can promote fatty acid oxidation by the activation of PPARα or the suppression of miR-122. SIRT6 can also directly modulate acyl-CoA synthetase long chain family member 5 (ACSL5) activity for fatty acid oxidation. SIRT6 also plays a critical role in the regulation of total cholesterol and low-density lipoprotein (LDL)-cholesterol through the regulation of SREBP2 and proprotein convertase subtilisin/kexin type 9 (PCSK9), respectively. Hepatic deficiency of Sirt6 in mice has been shown to cause hepatic steatosis, inflammation, and fibrosis, hallmarks of alcoholic and nonalcoholic steatohepatitis. SIRT6 can dampen hepatic inflammation through the modulation of macrophage polarization from M1 to M2 type. Hepatic stellate cells are a key cell type in hepatic fibrogenesis. SIRT6 plays a strong anti-fibrosis role by the suppression of multiple fibrogenic pathways including the transforming growth factor beta (TGFβ)-SMAD family proteins and Hippo pathways. The role of SIRT6 in liver cancer is quite complicated, as both tumor-suppressive and tumor-promoting activities have been documented in the literature. Overall, SIRT6 has multiple salutary effects on metabolic homeostasis and liver health, and it may serve as a therapeutic target for hepatic metabolic diseases. To date, numerous activators and inhibitors of SIRT6 have been developed for translational research.
Keywords: SIRT6; fatty acid oxidation; fibrosis; hepatocellular carcinoma; inflammation; lipogenesis; modulator.
Conflict of interest statement
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
SIRT6 controls hepatic lipogenesis by suppressing LXR, ChREBP, and SREBP1.Biochim Biophys Acta Mol Basis Dis. 2021 Dec 1;1867(12):166249. doi: 10.1016/j.bbadis.2021.166249. Epub 2021 Aug 21. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34425214 Free PMC article.
-
Qige Decoction attenuated non-alcoholic fatty liver disease through regulating SIRT6-PPARα-mediated fatty acid oxidation.Phytomedicine. 2025 Mar;138:156395. doi: 10.1016/j.phymed.2025.156395. Epub 2025 Jan 17. Phytomedicine. 2025. PMID: 39855055
-
Cytoplasmic SIRT6-mediated ACSL5 deacetylation impedes nonalcoholic fatty liver disease by facilitating hepatic fatty acid oxidation.Mol Cell. 2022 Nov 3;82(21):4099-4115.e9. doi: 10.1016/j.molcel.2022.09.018. Epub 2022 Oct 7. Mol Cell. 2022. PMID: 36208627
-
The ménage à trois of autophagy, lipid droplets and liver disease.Autophagy. 2022 Jan;18(1):50-72. doi: 10.1080/15548627.2021.1895658. Epub 2021 Apr 2. Autophagy. 2022. PMID: 33794741 Free PMC article. Review.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
Cited by
-
Effects of Portulaca oleracea (purslane) on liver function tests, metabolic profile, oxidative stress and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: a randomized, double-blind clinical trial.Front Nutr. 2024 Jul 29;11:1371137. doi: 10.3389/fnut.2024.1371137. eCollection 2024. Front Nutr. 2024. PMID: 39135554 Free PMC article.
-
Impaired SUMOylation of FoxA1 promotes nonalcoholic fatty liver disease through down-regulation of Sirt6.Cell Death Dis. 2024 Sep 14;15(9):674. doi: 10.1038/s41419-024-07054-1. Cell Death Dis. 2024. PMID: 39277582 Free PMC article.
-
GB1a Activates SIRT6 to Regulate Lipid Metabolism in Mouse Primary Hepatocytes.Int J Mol Sci. 2023 May 31;24(11):9540. doi: 10.3390/ijms24119540. Int J Mol Sci. 2023. PMID: 37298491 Free PMC article.
-
Recent Advances in the Discovery of SIRT1/2 Inhibitors via Computational Methods: A Perspective.Pharmaceuticals (Basel). 2024 May 8;17(5):601. doi: 10.3390/ph17050601. Pharmaceuticals (Basel). 2024. PMID: 38794171 Free PMC article. Review.
-
The diagnostic value and associated molecular mechanism study for fibroblast-related mitochondrial genes on keloid.Skin Res Technol. 2024 Sep;30(9):e70024. doi: 10.1111/srt.70024. Skin Res Technol. 2024. Retraction in: Skin Res Technol. 2025 Jun;31(6):e70188. doi: 10.1111/srt.70188. PMID: 39221860 Free PMC article. Retracted.
References
-
- Kim H.S., Xiao C., Wang R.H., Lahusen T., Xu X., Vassilopoulos A., Vazquez-Ortiz G., Jeong W.I., Park O., Ki S.H., et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12:224–236. doi: 10.1016/j.cmet.2010.06.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

